Preparation of spherical calcium carbonate by hypergravity reaction crystallization and carbonization
The common forms of calcium carbonate mainly include irregular shape, spindle shape, spherical shape, flake shape and cube shape, etc. Different forms of calcium carbonate have different application fields and functions. , solubility and large specific surface area, etc., have important applications in the fields of plastics, rubber, food and paper making.
At present, the main preparation methods of spherical calcium carbonate are metathesis method and carbonization method. Although the metathesis method can produce spherical calcium carbonate with regular morphology and good dispersion, the raw materials of this method are expensive and a large amount of impurity ions will be introduced, which is not suitable for industrial production. The carbonization method is the most commonly used method in the industry. The traditional carbonization method is mainly divided into the intermittent carbonization method and the continuous spray carbonization method. Although the carbonization method has low cost and can be produced on a large scale, the traditional carbonization method for preparing spherical calcium carbonate has problems such as uneven particle size distribution and low production efficiency.
The hypergravity reaction crystallization method is a new method of preparing nanomaterials, and its essence is to generate huge centrifugal force through high-speed rotation, simulating the environment of hypergravity field. The high-speed rotating packing rotor in the hypergravity reactor beats the liquid into liquid filaments, droplets or liquid films, and the specific surface area of the liquid increases sharply. 1 to 3 orders of magnitude, the micro-mixing and mass transfer processes are greatly enhanced, so the reaction time is shorter than the traditional carbonization method, and the product has the advantages of small particle size, narrow particle size distribution, high product purity, and more regular morphology. . Hypergravity reactors are widely used in the preparation of nanomaterials due to their good micro-mixing and mass transfer effects.
Spherical calcium carbonate is grown from vaterite in most cases, but vaterite, as a thermodynamically unstable crystal form, is difficult to exist stably in a humid environment and aqueous solution, and requires some special methods to obtain it stably. The research shows that the introduction of NH4+ during the carbonization reaction can not only inhibit the formation of calcite during the crystallization process, and facilitate the transformation of the crystal form of calcium carbonate to vaterite, but also the atmosphere of NH4+ can make the generated vaterite exist stably in the solution.
Different from NH4+, acidic amino acids will dissociate in solution and combine with Ca2+ to form a seed crystal template. Under the influence of the seed crystal template, the resulting calcium carbonate will also appear metastable crystal phase, and suitable amino acid The introduction will generate specific functions and modify the morphology during the crystallization of calcium carbonate.
Using inexpensive glutamic acid and ammonium chloride as additives, the controllable preparation of spherical calcium carbonate in a hypergravity field was studied, and the effects of the two additives in the synthesis of calcium carbonate were investigated. The results showed that:
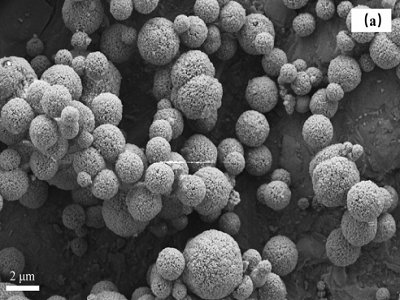
(1) Using the hypergravity reaction crystallization and carbonization method, the particle size can be obtained under the optimal conditions that L-glutamic acid and ammonium chloride are added at 4% and 20% calcium hydroxide, respectively, and the hypergravity factor is 161.0. Pure vaterite calcium carbonate with high sphericity of about 500nm.
(2) Before the reaction starts, L-glutamic acid and calcium ions in the solution form a template, which affects the nucleation and growth of calcium carbonate, and the abundant NH4+ in the solution during the reaction provides a good environment for the formation of vaterite, The high-speed cutting of the liquid by the hypergravity reactor prevents the possibility of excessive coating of calcium hydroxide raw materials, and realizes the controllable preparation of spherical calcium carbonate.
