Natural Zeolite Modification Technology and Its Application in Wastewater Treatment
Among many water treatment technologies, adsorption method has become an ideal wastewater treatment technology due to its advantages of simple operation, low energy consumption, good removal effect and high selectivity. The development of low-cost and high-efficiency adsorbents is the core of adsorption methods. Compared with other synthetic high-efficiency adsorbents, low-cost natural adsorbents have higher economic benefits and environmental protection value.
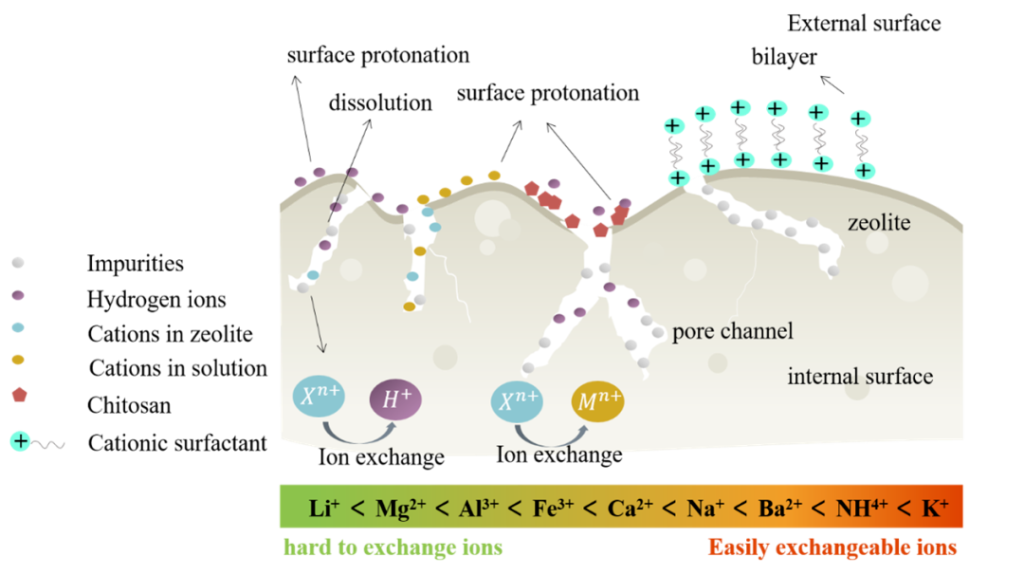
The abundant pores and channels in natural zeolites and the negative charge on the surface make them have good adsorption capacity for cations and little adsorption capacity for anions. This greatly limits the application of natural zeolites in the removal of anionic pollutants in water. For this reason, many studies have been carried out on the modification of natural zeolites in order to increase the affinity for anions. Surface modification is an effective way to increase the affinity of natural zeolites for anionic pollutants.
Different modification methods will have different effects on the physical and chemical properties of zeolite, such as changing the internal pore structure and size of zeolite, as well as hydrophilic and hydrophobic and surface functional groups. The main purpose of physical modification is to remove some impurities on the surface of zeolite and increase the specific surface area. The purpose of chemical modification is: (1) to remove impurities and dredge pore channels to facilitate the entry and transfer process of target substances, (2) to introduce new functional groups to change the surface properties of zeolite, such as hydrophobicity, thereby providing Novel binding sites for target pollutants.
Composite modification can achieve the purpose of synergistic modification by combining multiple modification methods. In order to better balance the preparation cost and removal effect, it is a better choice to improve the adsorption capacity of natural zeolite to anionic pollutants in water by means of compound modification.
There are still many challenges in the practical wastewater treatment of zeolites. For example, the pore size of natural zeolites usually belongs to the category of micropores, which are smaller than the radius of anions, which will hinder their migration and diffusion inside the zeolite, which is not conducive to the adsorption process. Moreover, the components in the actual wastewater are complex and changeable, and zeolites are easily affected by coexisting ions and pH values, resulting in poor adsorption effects and even structural damage. In addition, the saturated zeolite may be transformed into a new pollution source if it is not properly disposed of.
(1) The surface modification method will affect the physical and chemical properties of natural zeolite. Composite modification is an effective way to improve the anion adsorption performance of natural zeolite. For example, by introducing mesoporous materials to expand the pore size of zeolite and improve the diffusion efficiency of anions in the internal structure of zeolite. By introducing functional groups with affinity for target pollutants, the adsorption sites of zeolites can be enriched and the adsorption selectivity can be improved.
(2) Combining natural zeolite with other water treatment processes or materials can effectively improve its application potential in actual wastewater treatment. The pollution components in actual wastewater are complex and changeable, and the combined use of multiple materials/processes has become the mainstream way to improve the effect of actual wastewater treatment. Materials or combined processes containing natural/modified zeolites have been widely used in the treatment of wastewater, domestic sewage, rivers and lakes, etc. Natural zeolites and their modified forms have good application prospects in practical wastewater treatment.
(3) The modification and regeneration process of zeolite may involve toxic solvents, causing great harm to the environment and human health. A safe, pollution-free preparation and regeneration scheme should be sought, or a stable encapsulation method developed as a practical solution for final and safe disposal of zeolites.
