The key technical problems of ultrafine powder - dispersion and agglomeration

The agglomeration of ultrafine powder refers to the phenomenon that the original powder particles are connected to each other during the preparation, separation, processing and storage processes, and multiple particles form larger particle clusters. It is currently believed that there are three main reasons for the agglomeration of ultrafine powders: intermolecular forces causing agglomeration of ultrafine powders; electrostatic forces between particles causing agglomeration; and particle adhesion in the air.
1. Intermolecular forces cause ultrafine powder agglomeration
When the mineral material is ultrafine below a certain level, the distance between particles is extremely short, and the van der Waals force between particles is much greater than the gravity of the particles themselves. Therefore, such ultrafine particles tend to attract each other and agglomerate. Hydrogen bonds, adsorbed wet bridges and other chemical bonds on the surface of ultrafine particles can also easily lead to adhesion and aggregation between particles.
2. Electrostatic forces between particles cause agglomeration
During the ultrafine process of mineral materials, due to impact, friction and reduction in particle size, a large amount of positive or negative charges accumulate on the surface of new ultrafine particles. Some of the protrusions on the surface of these particles are positively charged and some are negatively charged. These charged particles are extremely unstable. In order to become stable, they attract each other and contact and connect with each other at the sharp corners, causing the particles to agglomerate. This process is The main force is electrostatic force.
3. Adhesion of particles in air
When the relative humidity of the air exceeds 65%, water vapor begins to condense on the surface of the particles and between the particles, and the agglomeration effect is greatly enhanced due to the formation of liquid bridges between the particles.
Dispersion of ultrafine powder
The dispersion of ultrafine powders mainly focuses on the dispersion state of particles in the gas phase medium and the dispersion state in the liquid phase.
Dispersion method in liquid phase: 1. Mechanical dispersion method. (The mechanical dispersion method is a method that uses mechanical energy such as external shear force or impact force to fully disperse nanoparticles in the medium. Mechanical dispersion methods include grinding, ordinary ball mill, vibration ball mill, colloid mill, air mill, mechanical stirring, etc.) 2. Chemical dispersion method 3. Ultrasonic method
Dispersion method in gas phase: 1. Dry and disperse 2. Mechanical dispersion (Mechanical dispersion refers to using mechanical force to break up the agglomeration of particles. Its necessary condition is that the mechanical force should be greater than the adhesion force between particles. Usually the mechanical force is caused by the strong turbulent movement of the airflow caused by the high-speed rotating impeller disc or the jet and impact of the high-speed airflow.) 3. Electrostatic dispersion
There are many modification methods for ultrafine powder, which are also very different from the previous mainstream methods. However, no matter which method is used, it is necessary to further study the modification principle of ultrafine powder and find a new modification method that is suitable for various modification requirements and can be applied to actual production.
Processing technology and application of fruit and vegetable powder

Processing technology of fruit and vegetable powder
1.Ultra-fine grinding technology
Generally refers to the processing of 0.1-10μm ultra-fine powder and corresponding classification technology. The particle size of the product particles is extremely small, the specific surface area increases sharply, and the cell wall breaking rate increases, thereby improving the physical and chemical properties of the material (dispersion, adsorption, dissolution properties, chemical activity, biological activity, etc.), expand the application scope of materials, and enhance the use effects of materials.
2. Bioenzymatic hydrolysis technology
For fresh fruits, vegetables and fungi, bioenzymatic treatment is used after crushing to break down the cell walls and dissolve nutrients.
3. Vacuum freeze drying
Vacuum freeze-drying technology is a new drying method that freezes water-containing materials into solids and uses the biochemical properties of water to dehydrate materials at low temperatures and achieve dryness under low-temperature and low-pressure conditions.
4. Spray drying technology
Spray drying is used to make powder. The raw material used is sauce-like liquid, which avoids the problem of difficult processing and molding. The drying process is completed instantaneously (a few seconds) at a temperature not higher than 100°C. Generally, the color, aroma, and taste of fruits are harmonious. The nutrients can be better protected and it is currently the best method for fruit and vegetable flour making.
5. Low temperature differential pressure puffing technology
The variable temperature pressure difference puffing drying technology is a combined drying technology that draws on hot air drying, vacuum expansion drying, etc. It absorbs the advantages of hot air drying and vacuum freeze drying, overcomes the shortcomings of vacuum low-temperature frying drying, and can produce products similar to The products processed by freeze drying belong to a new, environmentally friendly and energy-saving puffing and drying technology.
6. Screw extrusion technology
By means of the friction, extrusion and melting effect of the screw and barrel on the material, the purpose of transportation, compression and crushing, mixing, expansion and polymerization is achieved.
7.Microwave/vacuum technology:
Combines microwave drying and vacuum drying technologies. It accelerates water loss at low temperatures and is suitable for substances with high heat sensitivity. It is suitable for the production of vegetable powder, egg yolk powder and dehydrated grapes.
Application of fruit and vegetable powder in food
Fruit and vegetable powder can be applied to various fields of food processing, helping to increase the nutritional content of products, improve the color and flavor of products, and enrich product varieties.
Mainly used for: Pasta products, such as adding radish powder to noodles to make carrot noodles; Puffed foods, such as using tomato powder as a seasoning for puffed foods; Meat products, such as adding vegetable powder to ham sausage; Dairy products, For example, various fruit and vegetable powders are added to milk products; candy products, apple powder and strawberry powder are added during candy processing; baked products, such as onion powder and tomato powder are added during biscuit processing.
Using fruit and vegetable powder to make drinks does not affect the flavor of fresh fruits and vegetables; fruit powder can be made into fruit wine and fruit vinegar through fermentation, blending and filtration processes.
Candy, pastries, biscuits, bread and many other foods can add a certain proportion of fruit and vegetable powder during the production process, which can improve the nutritional structure of the product and make the product better in color, aroma and taste.
Fruit and vegetable powders contain pigments, pectin, tannins and other ingredients. Some specific fruits and vegetables also contain medicinal ingredients, from which valuable by-products can be extracted through biochemical pathways.
Fruit and vegetable juices are rich in a variety of vitamins and minerals. After proper processing, cyclodextrin and other substances are added to effectively embed and protect most of the nutrients in the fruit and vegetable juices, and at the same time, some nutrients are strengthened, and then Homogenize and vacuum freeze-dry to obtain nutritious fruit and vegetable powder.
Adding fruit and vegetable powder to food for infants, young children and the elderly can supplement vitamins and dietary fiber for a balanced diet.
Diversity and application fields of microcrystalline alumina ceramics

Microcrystalline alumina ceramics refers to alumina ceramic materials that use high-purity α-Al2O3 powder as the main raw material, are made through ceramic technology, the crystal grain size is less than 6 μm, and corundum is the main crystal phase.
Microcrystalline alumina ceramics are usually divided into two types: high purity type and ordinary type:
High purity microcrystalline alumina ceramics
High-purity microcrystalline alumina ceramics refer to alumina ceramic materials with an Al2O3 content of more than 99.9%. Its sintering temperature is as high as 1650~1990℃, and the transmission wavelength is in the range of 1~6μm. It uses its light transmittance and resistance to alkali metal corrosion and other properties, often used as high-pressure sodium lamp tubes.
Ordinary microcrystalline alumina ceramics
Ordinary microcrystalline alumina ceramics can be divided into 99, 95, 92, 90, 85 porcelain and other varieties according to the Al2O3 content (sometimes those with an Al2O3 content of 80% or 75% are also classified as ordinary alumina). Among them, 99 alumina ceramic materials are often used to make high-temperature crucibles, refractory furnace tubes and other special wear-resistant materials (such as ceramic bearings, ceramic seals and water valves). In the electronics industry, they can be used as integrated circuit substrates and high-end materials. Frequency insulating materials, commonly used in the chemical industry as catalyst carriers, etc.; 95, 92, and 90 alumina porcelain are mainly used as corrosion-resistant, wear-resistant materials and wear-resistant parts; 85 porcelain is often mixed with some talc, which improves the electrical properties With good mechanical strength, it can be sealed with niobium, tantalum and other metals and used as electronic vacuum device components.
Application fields of microcrystalline alumina ceramics
Non-metallic mineral deep processing industry
At present, billions of tons of non-metallic minerals are crushed and ground every year around the world, requiring a large amount of microcrystalline alumina ceramic grinding media and other various grinding media. Due to the excellent wear resistance of microcrystalline alumina ceramic grinding media and the requirements for high-quality ceramic products on grinding media, it will become an inevitable trend for microcrystalline alumina ceramic grinding media to gradually replace other grinding media in the future.
Electronic field
Microcrystalline alumina ceramics have excellent insulation properties and thermal stability, so they are widely used in the field of electronics and electrical appliances to manufacture electronic components, circuit boards, semiconductor packaging, etc. With the rapid development of the electronic industry, especially the microelectronics industry, the demand for alumina ceramic substrates continues to increase.
Petrochemical
Microcrystalline alumina ceramics, especially microcrystalline alumina ceramics with an alumina content of more than 97%, are typically used in oil and gas drilling equipment as nozzles, valve seats, regulating devices, pump accessories, drill bit accessories, etc. Works normally under high pressure, vibration environment, even in the presence of acids and alkalis.
Military field
Microcrystalline alumina ceramics also have many applications in the military field, such as ballistic armor for aircraft, vehicles and personnel.
Coal-fired power generation field
Microcrystalline alumina bricks and curved plates are successfully used as linings for coal-fired power generation equipment. This lining material is used for high-speed feeding of pulverized coal particles, burners, fly ash and residue treatment, etc., especially coal combustion The fly ash produced contains high amounts of quartz and different minerals and slag components, and their abrasive power is stronger than that of coal particles. Due to the different composition of fly ash, the pH value of mortar has a wide range (2.5-12) and is highly corrosive. Therefore, microcrystalline alumina products can be used as ideal materials for lining coal-fired power generation equipment.
Application Fields of Spherical Alumina Powder
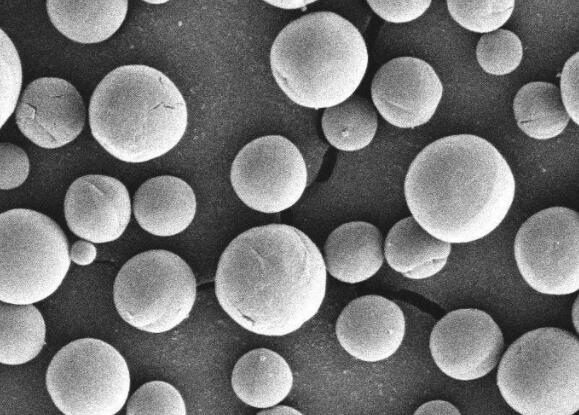
The unique physical and chemical properties of ultrafine spherical alumina make it widely used in bioceramics, surface protective layer materials, chemical catalysts and catalyst carriers, integrated circuit chips, aerospace, infrared absorption materials, and moisture-sensitive sensors.
The excellent performance of ultrafine spherical alumina products in many fields is closely related to the morphology and size of the raw powder particles. Regular morphology, small specific surface area, large packing density, good flow performance, high hardness and strength can greatly improve the application performance of the product.
Application fields of spherical alumina powder
1. Precision polishing abrasives
Alumina has gradually been widely used in industries such as precision processing and manufacturing due to its high hardness and good stability, especially in chemical mechanical polishing (CMP).
2.Special ceramic raw materials
The requirements for ceramic bodies are high density, small shrinkage deformation, and easy sintering. The size, morphology and dispersion of ceramic powder are important indicators to measure the performance of the powder. Among the many morphologies of powder, dispersed spherical micro-powder is better.
3. Other applications
Spherical alumina powder can be used as a support for porous alumina. Since the pores formed are relatively regular, it is easy to homogenize the entire support. Alumina powder for filling requires good fluidity, strong ability to combine with organic matter, and a spherical shape is preferred. Alumina is also the main raw material for three primary colors and long afterglow phosphors. In addition, it also has many applications in the fields of catalysts and catalyst carriers.
Preparation of ultrafine spherical alumina
With the rapid development of global industry, spherical alumina powder has been extensively studied in the past 10 years. The preparation of spherical alumina has become a hot topic in materials research.
Ball milling method
The ball milling method is the most common method for preparing ultrafine alumina powder. The rotation or vibration of the ball mill is usually used. The raw materials are impacted, ground and stirred by the abrasive, and the large particle size powder is refined into ultrafine powder.
Homogeneous precipitation method
The precipitation process in a homogeneous solution is a process in which crystal nuclei form, then aggregate and grow, and finally precipitate from the solution. If the concentration of the precipitant in the homogeneous solution can be reduced, or even generated slowly, it will be uniform. A large number of tiny crystal nuclei are generated, and the eventually formed fine precipitation particles will be evenly dispersed throughout the solution and will maintain an equilibrium state for a long time. This method of obtaining precipitation is called homogeneous precipitation.
Sol-emulsion-gel method
In order to obtain spherical powder particles, people use the interfacial tension between the oil phase and the water phase to create tiny spherical droplets, so that the formation and gelation of sol particles are limited to tiny droplets, and finally a spherical precipitation is obtained. Particles.
Drop ball method
The drop ball method is to drop alumina sol into an oil layer (usually paraffin, mineral oil, etc.), and form spherical sol particles by surface tension. Then the sol particles are gelled in an ammonia solution, and finally the gel particles are A method of drying and calcining to form spherical alumina.
Other methods
Spraying method: The essence of preparing spherical alumina by spraying method is to achieve phase transformation in a short time, and use the effect of surface tension to spherical the product. According to the characteristics of phase transformation, it can be divided into spray pyrolysis method and spray drying method. and injection melting.
Aerosol decomposition method: usually aluminum alkoxide is used as the raw material, and the aluminum alkoxide is easily hydrolyzed and pyrolyzed at high temperature, and the physical method of phase change is used to vaporize the aluminum alkoxide, and then contact with water vapor to hydrolyze and atomize , and then dried at high temperature or directly pyrolyzed at high temperature to achieve gas-liquid-solid or gas-solid phase transformation, and finally form spherical alumina powder.
Ultrafine spherical alumina powder has high added value and can bring greater social and economic benefits. In recent years, its demand has continued to grow. Therefore, the spheroidization of ultrafine alumina particles can greatly improve the application performance of its products and has great economic benefits. It is believed that the spheroidized alumina powder market will further expand!
How much do you know about medicinal talc?

In the pharmaceutical industry, talc powder has a wide and long history of use. It is often used as a lubricant and diluent in oral solid preparations such as tablets and capsules.
The main component of talc powder is hydrous magnesium silicate, which is mainly composed of magnesium oxide, silicon dioxide and a small amount of water.
(1) Structure of talc powder
Talcum powder has a flaky structure and belongs to the monoclinic crystal system. The crystals are flaky, with lamellae as the basic unit. Different lamellae are connected by weak van der Waals forces. When sheared by external forces, peeling between layers is easy to occur. , slipping phenomenon. Talcum powder particles are usually leaf-shaped or radial, colorless, tasteless and odorless, with excellent physical properties and insoluble in water.
(2) Physical and chemical properties of talc powder
Talcum powder is a white or off-white, sand-free fine powder with a pearly luster on its cleavage surface. It is odorless and tasteless, has a greasy feel, and is easy to adhere to the skin. It can be dissolved in water, dilute hydrochloric acid or 8.5% sodium hydroxide solution. Insoluble. The hardness is 1.0~1.5, the refractive index is 1.54~1.59, and the specific gravity is 2.7~2.8.
(3) Processing of talc powder
Raymond mill, mechanical impact crusher, jet mill and other equipment are commonly used for grinding talcum powder. High-pressure suspension roller mill and Raymond mill are suitable for processing talc powder with larger particle size, while ultra-fine grinding mill is mainly used for processing talc powder with smaller particle size.
After the medicinal talc is ground into powder, it needs to be flotated to remove various impurities, such as asbestos (tremolite), carbon, dolomite, iron oxide and various other aluminum salts and carbonate minerals , then made into fine powder, treated with dilute hydrochloric acid, washed with water, and then dried.
Application of talcum powder in preparation technology
(1) Used as a dispersant for volatile oils
Because talc powder has a certain adsorption capacity, it can adsorb volatile oil to the surface of its particles and disperse it evenly. It increases the solubility of volatile oil by increasing the contact area between volatile oil and liquid medicine.
(2) Cover with powder coating layer
In sugar coating, talc powder can be used to coat the powder coating layer. White talc powder that passes through a 100-mesh sieve is suitable. The dosage is generally 3% to 6%. It can not only eliminate edges and corners and facilitate coating, It can also improve the stability of sugar-coated tablets.
(3) Used as lubricant
Currently, talc powder is often used as a lubricant in the prescriptions of dispersible tablets, capsules, chewable tablets, effervescent tablets, and sustained-release tablets. Talcum powder can reduce the friction between drug powders and improve the fluidity of drug powders by filling the depressions on the surface of drug powders.
(4) Used as filter aid
Talcum powder is not easy to react with drugs and has certain adsorption capacity, so it can be used as a filter aid. Talcum powder activated at 115°C, when added to the medicinal solution while hot, can absorb a small amount of polysaccharides, mucus, and gum impurities without destroying the active ingredients of the medicine itself.
Application of talc powder as pharmaceutical excipients
(1) Used as a disintegrant for hydrophobic drugs
After talcum powder is added to the drug, because it is a hydrophilic substance, it can improve the hydrophilicity of the entire drug, making it easier for water to penetrate into the drug and make it easier to disintegrate. Therefore, talc powder can be used as a disintegrant to shorten the disintegration of the drug. time, especially for hydrophobic drugs.
(2) Used as anti-adhesive agent
Stickiness problem is a common problem in the coating process, which can lead to slow coating speed, longer production cycle, pellet adhesion, reduced yield, film damage, affecting drug release and other problems.
(3) Increase the critical relative humidity of the drug
For drugs that easily absorb moisture, talcum powder can be added to the prescription to improve the stability of the drug.
(4) Affecting the release of drugs
It has been reported in the literature that insoluble particles in functional coating formulations can affect drug release characteristics, but the results and mechanisms of action are different.
Development and application of high-performance boron nitride materials

As a new ceramic material with excellent performance and great development potential, boron nitride includes five isomers, namely hexagonal boron nitride (h-BN), cubic boron nitride (c-BN), fiber Zinc mineral boron nitride (w-BN), rhombohedral boron nitride (r-BN) and rhombic boron nitride (o-BN).
Applications of Boron Nitride
Current research on BN mainly focuses on its hexagonal phase (h-BN) and cubic phase (c-BN). The former has lubricity, thermal conductivity and good high-temperature performance; the latter is also in a thermodynamic equilibrium and stable state at normal temperature and pressure. The main application area of h-BN is as a raw material for the synthesis of cubic boron nitride.
Hexagonal boron nitride
Hexagonal boron nitride is a material with high temperature resistance, corrosion resistance, high thermal conductivity, high insulation and excellent lubrication properties. According to the current situation, simplifying the process, reducing production costs and increasing the service life of components are the current comparisons of this type of material. Active research directions. Main applications: crucibles, boats, liquid metal delivery pipes, rocket nozzles, high-power device bases, etc. for smelting evaporated metals. It can also be used as various material additives.
cubic boron nitride
Used as abrasive material. Small particles of cBN single crystal can be used as abrasive material. CBN abrasive tools use the action of a bonding agent to bond cBN abrasive grains into products with a certain geometric shape as a superhard material abrasive tool.
Used as tool material. PcBN overcomes the shortcomings of cBN single crystal, such as easy cleavage and anisotropy, and is mainly used to make tool materials. PcBN cutting tools are particularly suitable for high-speed cutting and can also be used for high-precision cutting. They have been widely used in CNC machine tools and are suitable for cutting high-hardness materials.
With the continuous advancement of science and technology and the increasing demand for applications, boron nitride has broad prospects for future development. Here are some possible trends:
Improve preparation efficiency: Improving preparation efficiency is one of the ways to achieve large-scale production of boron nitride, and developing more efficient and economical preparation methods is its development goal.
At present, the preparation efficiency of boron nitride is low, requires higher temperature and pressure conditions, and the preparation cycle is long. One of the future research directions is to develop more efficient and economical preparation methods to improve the preparation efficiency of boron nitride.
Develop new materials: In addition to conventional boron nitride materials, new materials such as two-dimensional boron nitride and porous boron nitride will receive attention. These new materials have unique structures and properties and are expected to be used in a wider range of fields.
Expand application fields: Boron nitride has been widely used in electronics, optoelectronics, materials science and other fields. Its excellent performance can expand more application fields in the future, such as biomedicine, environmental protection and other fields.
Improve performance and stability: The mechanical and chemical properties of boron nitride can be improved by controlling the crystal structure and purity to meet higher application requirements in the future.
Effect of ultrafine fly ash powder on cement properties

Fly ash is a small particle produced during the combustion process of coal-fired power plants. It is mainly composed of glass, minerals and carbon. Ultrafine powder refers to powder particles with a particle size less than 0.1 mm. In cement production, ultrafine fly ash powder can be used as an auxiliary cementing material to improve the performance of cement.
Effect of ultrafine fly ash powder on cement properties
1. Improve cement strength
Ultrafine fly ash powder can significantly improve the strength of cement. This is because the ultrafine fly ash powder has high activity and can react with the hydration products in the cement to form a denser structure, thus improving the strength of the cement. In addition, fly ash ultrafine powder can also fill the pores of cement, reduce the occurrence of cracks, and further enhance the strength of cement.
2. Improve cement fluidity
Fly ash ultrafine powder has good flow properties and can improve the fluidity of cement. Adding an appropriate amount of ultrafine fly ash powder to cement can reduce the viscosity of the mixture and improve its fluidity, making construction more convenient and faster.
3. Reduce cement hydration heat
Ultrafine fly ash powder can reduce the heat of hydration of cement. This is because the ultrafine fly ash powder can react with the minerals in the cement to form low-calorie compounds, thereby reducing the hydration heat of the cement. This is of great significance for the construction of large-volume concrete and can reduce the occurrence of temperature cracks.
4. Improve cement impermeability
Fly ash ultrafine powder can improve the impermeability of cement. This is because the ultrafine fly ash powder can react with the minerals in the cement to form a denser structure, reduce the generation of pores, and thus improve the impermeability of the cement. This is of great significance for projects such as basements that require waterproofing requirements.
Fly ash ultrafine powder is an industrial waste with high utilization value and can play an important role in cement production. By adding an appropriate amount of ultrafine fly ash powder, the properties of cement can be improved, increasing its strength, fluidity, impermeability and durability. At the same time, the application of ultrafine fly ash powder can also reduce cement production costs and environmental pollution, meeting the requirements of sustainable development.
Characteristics of conventional powders in the chemical industry

Characteristics of talc powder
Talcum powder, whose main component is hydrated magnesium silicate, is a white or off-white fine sand-free powder. It has excellent physical and chemical properties such as lubricity, fire resistance, acid resistance, insulation, high melting point, and chemical inertness.
Characteristics of kaolin clay
Kaolin, also known as dolomite, is a non-metallic mineral mainly composed of clay minerals of the kaolinite family, forming clay and clay rock.
In terms of chemical properties, kaolin has excellent electrical insulation properties, good acid solubility resistance, very low cation exchange capacity, high refractoriness and other physical and chemical properties.
Characteristics of mica powder
Mica powder is a non-metallic mineral whose main components are silica and aluminum oxide.
In terms of chemical properties, mica powder shows good acid and alkali corrosion resistance, high temperature resistance and other properties. In addition, plastic mica powder processed through special processes has the characteristics of high diameter-to-thickness ratio, high temperature resistance, acid and alkali resistance, and wear resistance. It is a natural functional powder filling material.
Characteristics of silica powder
Microsilica powder is a fine granular solid material with a particle size generally less than 1 micron. It is a new functional mineral raw material composed of natural microcrystalline quartz (a-quartz). It is mainly white or off-white.
Microsilica powder has a series of excellent properties: low thermal expansion coefficient, excellent dielectric properties, high thermal conductivity and good suspension performance.
Characteristics of aluminum hydroxide
In the chemical industry, aluminum hydroxide is mainly used as a flame retardant. It is not only flame retardant, but also prevents smoke, dripping, and toxic gases. Therefore, it has been widely used in electronics, chemicals, cables, plastics, rubber and other industries. In particular, ultrafine aluminum hydroxide has become the most widely used and widely used low-smoke, halogen-free material due to its multiple functions such as flame retardancy, smoke suppression, filling, and environmental protection.
Characteristics of alumina
Aluminum oxide, with the chemical formula Al2O3, is an inorganic substance. It is a compound with high hardness and a melting point as high as 2054°C. It is a typical ionic crystal and can be ionized at high temperatures.
Chemically, alumina is a highly hard material with a Mohs hardness of up to 9, which makes it widely used as a wear-resistant and corrosion-resistant material in many applications. Alumina has good thermal conductivity, and Al2O3 with high purity requirements is generally prepared by chemical methods.
In terms of industrial applications, aluminum oxide is widely used in the materials industry due to its high hardness, wear resistance and corrosion resistance.
Characteristics of barium sulfate
Barium sulfate is a colorless orthorhombic crystal or white amorphous powder with stable chemical properties and insoluble in water, acid, alkali or organic solvents. Barium sulfate is made from barite as the main raw material, and is processed through a series of processes such as mineral processing, mineral washing, and crushing.
Characteristics of diatomite
Diatomaceous earth is a naturally occurring inorganic mineral with colors such as white, off-white, gray and light gray brown, and has the characteristics of fine, loose, light and porous. It has very strong water absorption and permeability, so it is often used as a filler or anti-settling agent in paint, coating, rubber, plastic and other industries.
Diatomite also has good stability and is an important industrial material for heat insulation, grinding, filtration, adsorption, anticoagulation, demoulding, filling, carrier, etc.
Bentonite characteristics
Bentonite, also known as bentonite, bentonite or bentonite, is a non-metallic mineral whose main mineral component is montmorillonite.
The color of bentonite is usually white or light yellow, but due to changes in iron content, it may also appear light gray or light green.
Characteristics of transparent powder
Transparent powder is a new type of functional filler. It is a composite silicate. Its main component is a composite silicate containing magnesium, aluminum and calcium, which is an inorganic salt. Its characteristics are as follows:
1. High transparency
2. Good hardness and gloss
3. Low oil absorption
4. Good collapse resistance and less dust during use.
5. Ultra-transparent ultra-fine powder material is developed through the process of raw material selection-mixing-melting-rough grinding-fine grinding-grading.
Dry fine grinding for agrochemical applications

The reason why pesticide manufacturers develop specific components and dosage forms is to use the active ingredients at the right time and in the right amount when crops need protection, to effectively reduce factors that are detrimental to crop growth. Therefore, a plant protectant is essentially a mixture of different ingredients. These ingredients can basically be summarized into three major categories: active ingredients in the formula; fillers used to dilute the active substances, such as clay, talc, kaolin or silica; auxiliaries and additives used to improve the quality of the formula (such as stabilizers, Wetting agents, protective agents, defoaming agents, etc.).
In the pesticide production process, the first step is feeding and mixing; the second step is grinding. Through different types of grinding equipment shown below, the mixed material particles are ground and dispersed to the target fineness to meet the application requirements. After grinding, it goes through a screening process to prevent the possible presence of oversized particles. Finally, add additives or fillers that do not require grinding and perform dispersion and mixing again.
The reason why pesticide particles are required to be ultra-fine particles and have a narrow particle size distribution: the finer the active ingredient particles, the more effective they are, which means that a smaller amount can be used to achieve the same effect. This is beneficial for safety, environmental and economic reasons: reducing toxic effects on people in the spray area; reducing environmental pollution; reducing the use of the most expensive active ingredients in the formulation, thereby reducing pesticide production costs and increasing profits .
The narrow particle size distribution facilitates a simplified pesticide application procedure: the powder is dispersed in water before application on crops. The finer the particles, the more stable the suspension will be and no sedimentation will occur during operation. During the pesticide spraying process, the problem of large particles clogging the nozzles of the spraying system is effectively reduced.
Choosing the right mill is crucial, and ALPA offers different dry grinding technologies depending on the fineness and specifications required by the pesticide manufacturer.
Impact grinding machine CSM with classifying function
This type of classifying mill offers the possibility of achieving both grinding and classifying functions in one system. The CSM classifier is a combination of a fine impact classifier and a guide wheel classifier. Using two independent motor drives, one for the grinding disc and the other for the grading wheel, the CSM can precisely adjust the grading wheel speed to obtain a wide range of final product fineness from d97=9μm to 200μm. By utilizing the geometry of the classifier impeller and the air seal between the classifier wheel and the machine top cover, precise control of the upper limit of the particle size of the grinding material is ensured, thereby achieving fine classification.
Fluidized bed jet mill
This jet mill is suitable for ultra-fine grinding of materials of various hardnesses (soft to extremely hard). In the grinding area, the particles are driven by high-speed airflow to collide and grind with each other. There are no additional grinding parts. The dynamic classifier controls the maximum particle size. The air flow velocity at the nozzle outlet in the grinding chamber can reach 500 to 600 m/s. Because high grinding energy and impact speed can be generated in the fluidized bed, it is possible to achieve D50 fineness of 1 to 5 μm.
Due to such structural characteristics, the airflow mill has a very attractive feature: during the grinding process, there will be no temperature increase in the grinding chamber. The reason is that the heat generated when particles collide with each other is offset by the cooling phenomenon produced by the expanding compressed gas, so that the temperature in the grinding chamber remains constant and the active material molecules will not be destroyed.
Currently, pesticide production is of increasing strategic importance. There must be a re-evaluation to place greater emphasis on environmental constraints, both during the production of products and their use on agricultural crops. However, meeting the needs of the world's population remains a huge challenge. The role of chemical engineering is to produce pesticides in the best possible way, which requires selecting the most suitable grinding technology to achieve this.
Several impact ultra-fine grinding in industry process

The impact ultra-fine grinding process generally refers to the grinding and classification process to prepare particle size distribution d9, ≤10 micron. It can be divided into two types: dry method and wet method. The ultra-fine crushing unit operations (i.e. one-stage ultra-fine crushing) currently used in industry include the following types.
(l) Open circuit process. Generally, flat or disc type, circulating tube type and other airflow mills have self-grading function, so this open circuit process is often used. In addition, this process is often used for intermittent ultrafine grinding. The advantage of this process flow is that the process is simple. However, for ultra-fine grinders that do not have the function of self-classification, since there is no classifier in this process, qualified ultra-fine powder products cannot be separated in time, so the particle size distribution range of general products is wide.
(2) Closed-circuit process, which is characterized by a classifier and an ultra-fine grinder forming a closed-circuit system of ultra-fine crushing and fine classification. This process is often used in continuous powder operations of ball mills, stirring mills, high-speed mechanical impact mills, vibration mills, etc. Its advantage is that it can separate qualified ultrafine powder products in time, so it can reduce the agglomeration of fine particles and improve the efficiency of ultrafine crushing operations.
(3) The open circuit process with pre-classification is characterized by the fact that the materials are classified before entering the ultra-fine pulverizer. The fine-grained materials are directly used as ultra-fine powder products, and the coarse-grained materials then enter the ultra-fine pulverizer for crushing. When the feed contains a large amount of qualified ultrafine powder, using this process can reduce the load of the crusher, reduce the energy consumption per unit of ultrafine powder product, and improve the efficiency of the operation.
(4) Closed-circuit process with pre-grading. This process is essentially a combination of two processes. This combined operation not only helps improve crushing efficiency and reduce energy consumption per unit product, but also controls the particle size distribution of the product. This process can also be simplified to only one grader, that is, pre-grading and inspection grading are combined into the same grader.
(5) Open circuit process with final classification. The characteristic of this crushing process is that one or more classifiers can be installed after the crusher to obtain two or more products with different fineness and particle size distribution.
(6) With pre-grading and final grading open circuit process, this process is essentially a combination of two processes. This combined operation can not only pre-separate some qualified fine-grained products, but also reduce the load on the crusher, and the final classification equipment can obtain two or more products with different fineness and particle size distribution.
The number of crushing stages mainly depends on the particle size of the raw materials and the required product fineness. For raw materials with relatively coarse particle sizes, a process of fine crushing or fine grinding and then ultra-fine crushing can be used. Generally, the raw materials can be crushed to 200 mesh or 325 mesh and then an ultra-fine crushing process can be used; for product particle size requirements For materials that are very fine and easy to agglomerate, a multi-stage ultra-fine crushing process in series can be used to improve operating efficiency. However, generally speaking, the more crushing stages, the more complex the process flow and the greater the engineering investment.
In terms of grinding methods, ultra-fine grinding processes can be divided into three types: dry (one or more stages) grinding, wet (one or more stages) grinding, and dry-wet combined grinding. The following introduces several typical ultra-fine grinding process flows.



