Aluminum hydroxide: Why can't it be used directly?

Inorganic amphoteric hydroxides—aluminum hydroxide (Al(OH)3, ATH)—possess highly efficient flame retardant, smoke suppressant, and filling properties. Upon thermal decomposition, it does not produce toxic or corrosive gases and can be used as a flame retardant filler in polymeric organic materials. Currently, the use of ATH as a flame retardant is increasing year by year, and ATH has become the most important inorganic flame retardant globally.
Modification First, Then Flame Retardancy
Generally, manufacturers typically fill flammable materials with powdered aluminum hydroxide (ATH) or coat the surface of flammable materials with a flame-retardant coating containing ATH to improve the flame-retardant properties of polymeric organic materials.
Furthermore, because ATH contains three hydroxyl groups (-OH), its surface is asymmetrical and highly polar. The surface hydroxyl groups exhibit hydrophilic and oleophobic properties, making it prone to agglomeration when added to polymeric organic materials, directly affecting the material's mechanical properties.
Therefore, aluminum hydroxide needs to be surface modified before use.
Surface Modification of Aluminum Hydroxide
Surface modification is one of the key technologies for optimizing the properties of inorganic powder materials, playing a crucial role in improving the application performance and value of inorganic powders. Surface modification of inorganic particles refers to the adsorption or encapsulation of one or more substances on the surface of inorganic particles, forming a core-shell composite structure. This process is essentially a composite process of different substances.
Types and Characteristics of Modifiers
There are many types of powder surface modifiers, but there is no standard classification method. Modifiers for inorganic powder modification are mainly divided into two categories: surfactants and coupling agents.
(1) Coupling Agents
Coupling agents are suitable for various composite material systems of organic polymers and inorganic fillers. After surface modification with coupling agents, the inorganic material's compatibility and dispersibility with the polymer are increased. The surface of the inorganic material changes from hydrophilic and oleophobic to oleophilic and hydrophobic, increasing its affinity with the organic polymer.
Coupling agents are diverse and can be classified into four main categories based on their chemical structure and composition: organic complexes, silanes, titanates, and aluminates.
(2) Surfactants
Surfactants are substances that can significantly alter the surface or interfacial properties of a material when used in very small amounts. They include anionic, cationic, and nonionic surfactants, such as higher fatty acids and their salts, alcohols, amines, and esters. Their molecular structure is characterized by a long-chain alkyl group at one end, similar to polymer molecules, and polar groups such as carboxyl, ether, and amino groups at the other end.
How can the modification effect be determined?
Is modified aluminum hydroxide reliable? How reliable is it? This requires evaluating and characterizing the modification effect.
Currently, the flame-retardant effect of aluminum hydroxide flame retardants can be evaluated through direct methods such as testing the material's oxygen index, vertical and horizontal flammability index, smoke production, thermogravimetric analysis, and mechanical properties during combustion; or indirectly by measuring powder absorbance, activation index, and oil absorption value to indirectly test its modification effect.
(1) Absorbance
Unmodified ATH has hydrophilic and oleophobic hydroxyl groups on its surface, allowing it to dissolve in water or settle freely to the bottom. After modification, the surface of ATH becomes hydrophilic and oleophobic, with surface properties completely opposite to the unmodified form. It cannot dissolve or settle to the bottom and can only float on the surface. However, modified ATH can dissolve or precipitate well in oils (such as liquid paraffin).
(2) Activation Index
Unmodified ATH has very strong polarity due to the nature of its surface hydroxyl groups (-OH), allowing it to dissolve or settle freely in water with similar properties. After modification, ATH has a layer of lipophilic groups attached to its surface, with surface hydroxyl groups (-OH) encapsulated within. The better the modification effect, the higher the lipophilic group coverage rate of the ATH surface, and the more modified ATH floats on the water surface.
(3) Oil Absorption Value
Measuring the oil absorption value requires adding castor oil to ATH and stirring. Before modification, ATH, due to its hydrophilic and oleophobic properties, requires more castor oil to form spheres. After surface modification, it becomes hydrophilic and oleophobic, improving the dispersibility of ATH in the polymer and reducing voids formed by powder agglomeration.
Understanding Super Strong Materials—NdFeB

Sintered NdFeB, as the earliest preparation process and the most universally applicable, has driven the rapid development of rare earth permanent magnet materials. Sintered NdFeB, with its strong magnetic anisotropy and low-cost raw material input, has become a research target for many countries. Sintered NdFeB permanent magnet materials utilize powder metallurgy. The smelted alloy is made into powder and pressed into a compact in a magnetic field. The compact is then sintered in an inert gas or vacuum to achieve densification. Furthermore, to improve the coercivity of the magnet, aging heat treatment is usually required. The process flow is as follows: raw material preparation → smelting → powder preparation → pressing → sintering and tempering → magnetic testing → grinding → machining → electroplating → finished product.
Unlike sintered NdFeB, the individual powder particles of bonded magnets need to have sufficiently high coercivity. Once the multiphase structure and microstructure required for high coercivity are severely damaged during the powder preparation process, it will be impossible to produce good bonded magnets. Therefore, by using the method of melt-spinning rapid quenching magnetic powder, the hot molten alloy is first poured or sprayed onto a high-speed rotating water-cooled copper wheel to form a thin strip with a thickness of 100 μm.
The manufacture of hot-pressed/hot-deformed magnets requires starting with rapidly quenched Nd-Fe-B magnetic powder, rather than directly using cast alloys. By employing over-quenching (rapid cooling) conditions, finer grains, or even amorphous magnetic powder, are prepared. During hot pressing and hot deformation, the grains are heated and grown to near single-domain size, thus achieving high coercivity in the final magnet. The hot pressing process involves placing the magnetic powder in a mold and applying pressure at high temperature to force it into an isotropic, solid-density magnet.
Application
Permanent Magnet Motors
In permanent magnet motors, the use of permanent magnets for excitation not only reduces power consumption and saves energy, but also improves motor performance.
Magnetic Machinery
Magnetic machinery operates using the repulsive force of like poles or the attractive force of unlike poles in magnets. This requires permanent magnets with high remanence and high intrinsic coercivity. Furthermore, due to the principle of attraction between unlike poles, magnetic drives can be constructed using non-contact transmission, offering advantages such as no friction and noise. Therefore, high-performance Nd-Fe-B magnets are widely used in drive components of mining machinery, magnetic bearings in gyroscopes and turbines in satellites and spacecraft, and rotor bearings in centrifugal pumps for assisting cardiac function in medical equipment.
Aerospace
Rare-earth permanent magnet materials are indispensable for rocket launches, satellite positioning, and communication technologies. High-performance sintered Nd-Fe-B is particularly useful in microwave transmitting/receiving systems for radar. Utilizing the combined effect of a constant magnetic field and an alternating microwave magnetic field, ferromagnetic resonance occurs, allowing for the fabrication of microwave circulators, isolators, etc. Consumer Electronics
3C consumer electronics has always been an important downstream industry for sintered NdFeB. Sintered NdFeB possesses characteristics such as high magnetic energy product, which aligns with the miniaturization, lightweighting, and thinning trends in 3C consumer electronics products. It is widely used in electronic components such as VCMs, mobile phone linear motors, cameras, headphones, speakers, and spindle drive motors.
Neodymium iron boron waste recycling: an unmissable treasure trove
Neodymium iron boron (NdFeB) permanent magnets are widely used in wind power generation, new energy vehicles, and electronic products due to their excellent magnetic properties, earning them the title of "King of Magnets." However, the scrap rate in the NdFeB magnet production process is as high as 30%, and coupled with their limited lifespan, this results in a large amount of NdFeB waste.
These wastes contain up to 30% rare earth elements, far exceeding the content of primary rare earth ores, making them a highly valuable secondary resource. Efficiently recovering rare earth elements from NdFeB waste is crucial for ensuring rare earth resource security, reducing environmental pollution, and promoting sustainable development.
Characteristics and Sources of NdFeB Waste
NdFeB waste mainly originates from scraps, defective products, and retired electronic products containing magnets during the magnet manufacturing process. Its chemical composition is complex; in addition to the main rare earth elements Nd and Pr, elements such as Dy and Tb are often added to improve coercivity, and elements such as Co, Al, and Cu are added to improve overall performance. Based on rare earth element (REE) content, NdFeB waste can be classified into three categories: low rare earth (REEs < 20%), medium rare earth (20%–30%), and high rare earth (> 30%).
Currently, the recycling processes for NdFeB waste are mainly divided into pyrometallurgical, hydrometallurgical, and novel recycling technologies.
(I) Pyrometallurgical Recycling Processes
Pyrometallurgical recycling separates rare earth elements from iron through high-temperature reactions. The main methods include selective oxidation, chlorination separation, liquid alloying, and slag-metal fusion separation.
Selective oxidation is based on the fact that rare earth elements have a much higher affinity for oxygen than iron. At high temperatures, rare earth elements are selectively oxidized to form oxides, which are then separated from metallic iron. Nakamoto et al. successfully prepared mixed rare earth oxides with a purity exceeding 95% and a recovery rate exceeding 99% by precisely controlling the oxygen partial pressure.
Chlorination separation utilizes the strong affinity between rare earth elements and chlorine. Chlorinating agents such as NH4Cl, FeCl2, or MgCl2 are used to convert rare earth elements into chlorides before separation. Uda used FeCl2 as a chlorinating agent, reacting at 800℃, achieving a rare earth recovery rate of 95.9% and a product purity exceeding 99%.
The liquid alloying method utilizes the difference in affinity between rare earth elements and iron for other metals to achieve effective enrichment and separation of rare earth elements and iron. Rare earth element Nd can form various low-melting-point alloys with Ag, Mg, etc.
The slag-metal separation method is based on the characteristic that rare earth elements in NdFeB waste more readily combine with oxygen. All the metals in the NdFeB waste are converted into metal oxides. Simultaneously, under the high temperature of a slagging agent, the iron oxides are converted into metallic Fe by controlling the reducing conditions.
(II) Wet Recovery Process
Wet recovery is currently the most widely used method, mainly including the total dissolution method, hydrochloric acid preferential dissolution method, double salt precipitation method, and solvent extraction method.
(III) New Recycling Processes
New recycling technologies aim to solve the problems of high energy consumption and high pollution associated with traditional methods, including hydrogen explosion, bioleaching, and electrochemical methods.
Comparison of Different Recycling Processes and Environmental Impact
Pyrometallurgical processes have short flow rates and large processing capacities, but high energy consumption and difficulty in separating single rare earth elements; hydrometallurgical processes have high recovery rates and high product purity, but high acid consumption and high wastewater treatment costs; newer processes such as bioleaching and electrochemical methods are environmentally friendly, but are mostly in the laboratory stage and have not yet been applied on a large scale.
In terms of environmental impact, traditional recycling processes often use strong acids, strong alkalis, and high temperatures, generating large amounts of waste liquid and waste gas, increasing the environmental burden. Therefore, developing green and low-consumption recycling processes is crucial.
NdFeB waste recycling is a key way to alleviate rare earth resource shortages and reduce environmental pollution. Through technological innovation and policy guidance, the NdFeB recycling industry will develop towards greening, low cost, short processes, and high recovery rates, injecting new impetus into sustainable development.
Application and development of inorganic powder materials in the rubber industry

Rubber is widely used in transportation, machinery, electronics, defense, and other sectors of the national economy. However, rubber also has its own significant drawbacks, such as weak intermolecular forces, large free volume, and poor self-crystallization ability, resulting in low strength and modulus, and poor wear resistance in rubber materials. Therefore, it is necessary to add inorganic non-metallic fillers to meet the requirements of these applications.
Generally speaking, inorganic non-metallic fillers in rubber mainly serve the following functions: reinforcement, filling (increasing volume) and cost reduction, improving processing performance, regulating vulcanization characteristics, and imparting special functions.
Commonly Used Inorganic Non-metallic Mineral Fillers in Rubber
(1) Silica
Silica is currently the second most widely used reinforcing agent in the rubber industry after carbon black. The chemical formula of silica is SiO2·nH2O. Its particle structure contains many voids. When these voids are in the range of 2nm-60nm, they easily combine with other polymers, which is the main reason why silica is used as a reinforcing agent. As a reinforcing agent, silica can greatly improve the wear resistance and tear resistance of materials. It can also significantly improve the mechanical properties of tires and is widely used in vehicles, instruments, aerospace, and other fields.
(2) Light Calcium Carbonate
Light calcium carbonate is one of the earliest and most widely used fillers in the rubber industry. Large amounts of light calcium carbonate added to rubber can increase the volume of the product, thereby saving expensive natural rubber and reducing costs. Light calcium carbonate filling rubber can achieve higher tensile strength, wear resistance, and tear strength than pure rubber vulcanizates. It has a significant reinforcing effect in both natural and synthetic rubber, and can also adjust consistency. In the cable industry, it can provide a certain degree of insulation. (3) Kaolin
Kaolinite is a hydrous aluminosilicate, a common clay mineral. Its practical application in rubber enhances the rubber's elasticity, barrier properties, elongation, and flexural strength. Adding modified kaolinite to styrene-butadiene rubber (SBR) significantly improves the rubber's elongation, tear strength, and Shore hardness, while also extending its service life.
(4) Clay
Clay can be added during tire manufacturing, depending on the production process requirements. Clay is used as a filler to reduce costs. However, it must be activated clay to facilitate bonding with rubber. Activated or modified clay can partially replace carbon black in the formulation.
Studies show that as the amount of clay increases, the hardness, 300% tensile stress, and tensile strength of the rubber compound decrease slightly, but this can be compensated for by adjusting the vulcanization system. When used in tread formulations, after system optimization, it can also reduce rolling resistance.
(5) Barium Sulfate
It can effectively enhance the anti-aging and weather resistance of rubber products such as tire rubber and belts. In addition, it can improve the surface smoothness of rubber products. As a powdered rubber filler, it can not only improve the powder application rate, but also has obvious advantages in terms of economic cost.
(6) Talc
Talc powder is usually divided into general industrial talc powder and ultrafine talc powder. The former, as a rubber filler, does not play a reinforcing role and has a negligible effect on improving the physical properties of rubber. Therefore, general industrial talc powder is often used as a separating agent. Ultrafine talc powder, on the other hand, has a good reinforcing effect. If it is used as a rubber filler, the tensile strength of the rubber itself is equal to the effect produced by silica.
(7) Graphite
Graphite belongs to the lamellar silicate non-metallic minerals and has good thermal conductivity, electrical conductivity, and lubricity. Using graphite as a rubber filler involves a similar process to that used for montmorillonite, where graphite is broken down into nano-sized particles using a special technique. When these nanoparticles combine with the rubber matrix, various functional properties of the rubber are improved. For example, electrical conductivity, thermal conductivity, airtightness, and mechanical properties are all significantly enhanced.
Types and Applications of Powder Spheroidization Technology
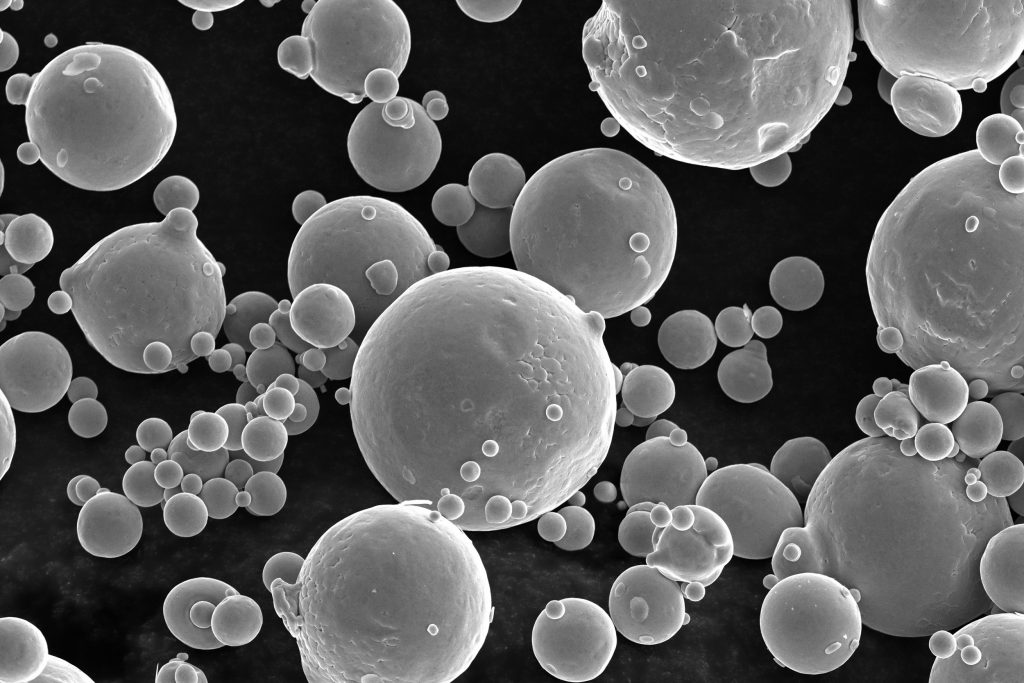
Powder spheroidization technology, an indispensable component of modern industry and science, can improve the surface characteristics and physical properties of powders, optimize material performance, and meet multifunctional requirements. Currently, powder spheroidization technology has penetrated numerous fields, including pharmaceuticals, food, chemicals, environmental protection, materials, metallurgy, and 3D printing.
Spherical powder preparation technology involves multiple disciplines, including expertise in chemistry, materials science, and engineering. Below, we will explore the various technologies involved in powder spheroidization.
Mechanical Shaping Method
Mechanical shaping methods primarily utilize a series of mechanical forces, such as collision, friction, and shear, to plastically deform and adsorb particles. Continuous processing results in denser particles, and sharp edges are gradually smoothed and rounded by the impact force. Mechanical shaping methods utilize high-speed impact mills, media stirred mills, and other pulverizing equipment to produce fine powder materials. Combined with dry and wet grinding, these methods yield powder materials with finer particle size, narrower particle size distribution, and a certain spheroidization rate.
Mechanical shaping is widely used in the spheroidization and shaping of natural graphite, artificial graphite, and cement particles. It is also suitable for crushing and pulverizing brittle metal or alloy powders. Mechanical shaping utilizes a wide range of low-cost raw materials, fully utilizing existing resources. It offers advantages such as simplicity, environmental friendliness, and industrial scalability. However, this method is not very selective in terms of materials, and cannot guarantee the sphericity, tap density, and yield of the processed particles. Therefore, it is only suitable for producing spherical powders with lower quality requirements.
Spray Drying
Spray drying involves atomizing a liquid substance into droplets, which are then rapidly evaporated in a hot air stream, solidifying into solid particles. The advantages of spray drying are its simplicity and ease of controlling product properties. It is primarily used in the fields of military explosives and batteries.
Gas-Phase Chemical Reaction
Gas-phase chemical reaction uses gaseous raw materials (or evaporates solid raw materials into a gaseous state) to produce the desired compound through a chemical reaction. This compound is then rapidly condensed to produce ultrafine spherical powders of various substances.
Hydrothermal Method
The hydrothermal method utilizes a reactor under high temperature and pressure conditions, using water or an organic solvent as the reaction medium for a chemical reaction. Particle size can be effectively controlled by adjusting parameters such as the hydrothermal temperature, hydrothermal time, pH, and solution concentration.
Precipitation Method
The precipitation method combines metal ions with a specific precipitant through a chemical reaction in a solution, generating tiny, semi-solid colloidal particles and forming a stable suspension. Subsequently, by further adjusting precipitation reaction conditions, such as static aging, slow stirring, or changing the solution environment, these colloidal particles gradually aggregate and grow toward spherical shape, forming a primary spherical precipitate. The resulting precipitate is then dried or calcined to ultimately produce a spherical powder material.
Sol-Gel Method
The sol-gel method typically involves three stages: sol preparation, gel formation, and spherical powder formation. Heat treatment can further improve the structure and properties of the spherical powder, enabling precise control of the particle size and morphology.
Microemulsion Method
The microemulsion method is a liquid-liquid two-phase system preparation method. This method involves adding an organic solvent containing a dissolved precursor to an aqueous phase to form an emulsion containing tiny droplets. Spherical particles are then formed through nucleation, coalescence, agglomeration, and heat treatment. Microemulsion methods are widely used in the preparation of nanoparticles and organic-inorganic composite materials.
Plasma Spheroidization
With the rapid development of high-tech and the urgent need for new nanomaterials and novel preparation processes, the research and application of plasma chemistry are gaining increasing attention. Plasma spheroidization, characterized by high temperature, high enthalpy, high chemical reactivity, and controllable reaction atmosphere and temperature, is ideal for producing high-purity, small-particle spherical powders.
Other methods include deflagration, Gas Combustion Flame Pelletization, Ultrasonic Atomization, Centrifugal Atomization, wire cutting, punching, and remelting, and pulsed micropore spraying.
How to modify the surface of silicon nitride powder?
![]()
Surface modification of silicon nitride powder primarily involves treating the surface of the powder through various physical and chemical methods to improve the physical and chemical properties of the particles.
Surface modification can reduce the mutual attraction between powder particles, allowing for better dispersion of the powder in the medium and improving the dispersibility of the powder slurry. It can also enhance the surface activity of the silicon nitride powder, increasing its compatibility with other substances and thus developing new properties.
The main principle of powder surface modification is that the interaction between the powder and the surface modifier enhances the wettability of the powder surface and improves its dispersion in aqueous or organic media.
1. Surface Coating Modification
Surface coating modification technology utilizes physical or chemical adsorption to uniformly attach the coating material to the surface of the coated object, forming a uniform and complete coating layer. The coating layer formed during the coating process is typically a monolayer.
Coating modification is generally categorized as inorganic and organic. Inorganic coating primarily involves depositing appropriate oxides or hydroxides on the surface of ceramic particles to modify the powder, but this modification only affects physical properties. Organic coating, on the other hand, involves selecting organic substances as coating materials. These organic substances bond with groups on the surface of the powder particles and selectively adsorb onto the surface, imparting the properties of the coating layer to the powder.
This modification technology offers low cost, simple steps, and easy control, but the resulting results are often limited.
2. Surface Acid and Alkali Treatment
Ceramic molding processes generally require ceramic slurries with high solids content and low viscosity. The charge density on the powder surface significantly influences the rheological and dispersibility of the slurry. Washing the ceramic powder surface (acid and alkaline treatments) can alter the surface charge properties of the powder. As the name suggests, this modification method involves thoroughly mixing and washing the silicon nitride powder with acid or alkaline solutions of varying concentrations.
At the same time, alkaline treatment at a certain concentration can also react with the surface of ceramic powders. Research by Wang Yongming et al. has shown that alkaline washing can reduce the silanol content on the surface of silicon carbide powder, lowering its degree of oxidation, altering the electrostatic repulsion between particles, and improving the rheological properties of the slurry.
3. Dispersant Modification
Based on the differences between different types of ceramic powders, selecting an appropriate dispersant or designing a new one plays a key role in increasing the solid content of the ceramic slurry. The type and amount of dispersant added can significantly alter the effect on ceramic properties.
Dispersants generally have both hydrophilic and hydrophobic structures, and it is through the interaction between these hydrophilic and hydrophobic groups that they adjust the dispersion properties of the ceramic slurry. Dispersants include surfactants or polymer electrolytes, with surfactants including cationic and anionic surfactants.
Polymer electrolytes include polyvinyl sulfonic acid, polyacrylic acid, polyvinyl pyridine, and polyethyleneimine. Dispersants can undergo adsorption reactions with the powder surface, including chemical and physical adsorption, leveraging interparticle forces (van der Waals forces and electrostatic repulsion) and the potential for steric effects.
4. Surface Hydrophobicity Modification
Surface hydrophobicity modification involves converting the hydroxyl groups in ceramic powder into hydrophobic groups, such as hydrocarbon groups, long-chain alkyl groups, and cycloalkyl groups. These organic groups bind to the ceramic powder surface, exerting a strong hydrophobic effect, enabling better dispersion in the dispersion medium and preventing agglomeration.
When polymers are grafted onto the surface of silicon nitride powder, the long polymer chains attach to the powder surface, while the hydrophilic chains at the other ends extend into the aqueous medium. Throughout the dispersion process, the powder particles experience both interparticle repulsion and steric hindrance created by the long polymer chains, resulting in better slurry dispersion.
Kaolin's four innovative application areas and prospects

Kaolin, a 1:1 layered silicate mineral, boasts numerous properties, including dispersibility, plasticity, sinterability, refractory properties, ion exchangeability, and chemical stability, making it widely used in various industrial fields. Currently, kaolin's applications are primarily concentrated in traditional industries such as ceramics, papermaking, and refractories.
1. High-Performance Composites
The application of kaolin in composites can improve the surface properties (such as adsorption capacity) of materials.
The benefits of kaolin in composites include enhancing adsorption, enhancing electrical properties, improving thermal stability/fire resistance, and improving mechanical stability. However, practical applications still present challenges, such as insufficient dispersibility and interfacial compatibility of kaolin in composites, which may limit its effectiveness.
Future research directions include developing more efficient and green kaolin surface modification technologies to improve its dispersibility and compatibility with matrix materials; exploring the design of multifunctional kaolin-based composites to meet the needs of specific applications, such as energy harvesting, wastewater treatment, and fire safety; and further increasing kaolin's specific surface area and number of active sites through nanoscale processing and molecular manipulation, thereby enhancing its performance. Furthermore, efforts should be made to promote low-cost and environmentally friendly production processes for kaolin composites, and to integrate intelligent manufacturing technologies to achieve large-scale application.
2. Porous Materials: Molecular Sieve Field
Molecular sieves are materials with an ordered pore structure that selectively adsorb different molecules. They are widely used in oil refining, petrochemicals, agriculture, and water treatment. Kaolin, a common and inexpensive natural mineral rich in silica and alumina, can be directly used to synthesize zeolite molecular sieves. Compared with traditional and potentially toxic silicon and aluminum sources, kaolin is not only environmentally friendly but also reduces costs and simplifies the synthesis process.
Kaolin not only activates silicate and alumina activity through simple pretreatments such as calcination and acid leaching, but also further enhances molecular sieve performance through templating agent manipulation and temperature optimization.
3. Biomedicine
Kaolin is a type of nanosilicate clay mineral characterized by excellent biocompatibility, high specific surface area, chemical inertness, colloidal properties, and thixotropy. In the biomedicine field, research is gradually shifting from basic drug carrier applications to more complex biomedical applications such as gene therapy and 3D bioprinting. Kaolin's applications have expanded from simple physical support and drug release to complex systems promoting cell growth and gene delivery.
4. Energy Storage
Energy storage has always been a hot topic. Seeking efficient and sustainable energy storage solutions is one of the key paths to addressing global energy challenges. Kaolin, with its unique structure and multifunctionality, has become an ideal candidate for energy storage. Kaolin is used in a variety of energy storage devices such as lithium-ion batteries, supercapacitors, and microbial fuel cells.
The future application prospects of kaolin are as follows:
a. Research and development of innovative materials will focus on kaolin nano-processing and surface modification technologies, aiming to enhance its performance in electronics, energy storage, and other fields. For example, kaolin-based nanocomposites can be developed by combining them with polymers or carbon-based materials to improve mechanical strength and conductivity.
b. Kaolin has the potential to provide solutions to environmental issues such as water treatment and soil remediation, particularly in the removal of heavy metals and adsorption of pollutants.
c. The integration of interdisciplinary technologies will promote the innovative application of kaolin in the biopharmaceutical field, integrating biotechnology to develop drug delivery systems or bioactive scaffolds.
d. With the increasing market demand for environmentally friendly materials, companies should strengthen collaboration with R&D institutions to transform innovative discoveries into competitive products, such as high-temperature, durable kaolin ceramics or lightweight composites.
e. With the global emphasis on sustainable development, policy support and economic feasibility will influence the direction of kaolin R&D and application. Therefore, the industry needs to closely monitor resource availability and cost optimization, while strengthening risk management and enhancing global competitiveness to cope with the complex international environment.
SDS-modified barium sulfate for cosmetic use

Cosmetic opacifiers are key ingredients for achieving effects such as concealing blemishes and brightening skin; their dispersibility and stability directly affect product performance and shelf life.
Barium sulfate is widely used in cosmetics due to its high refractive index, good opacity, and chemical stability. However, its tendency to agglomerate limits its application in cosmetics.
This study investigates the dispersibility and stability of barium sulfate in cosmetic matrices by preparing ultrafine barium sulfate using ball milling, and optimizing surface modification and dispersion processes.
1. Modification Methods
(1) Pretreatment of Barium Sulfate
Industrial-grade barium sulfate was dried and sieved through a 200-mesh screen in batches. For each batch, 100g of barium sulfate was mixed with 0.5g of stearic acid on a two-roll mill for 3 min. The rolls were then adjusted to the minimum gap and passed through 6 times, followed by a final pass with a 2mm gap, completing the initial mixing. The mixed barium sulfate was dried at 80°C for 4h to obtain the pretreated product.
(2) Surface Modification
Using 100 parts of the base formulation, different proportions of the pretreated barium sulfate were added and subjected to surface modification at 60°C. During modification, 1.5 parts of sodium dodecyl sulfate were added, and the mixture was thoroughly mixed. The rolls were adjusted to the minimum gap and passed through 6 times before being flattened, yielding the modified barium sulfate.
(3) Preparation of Dispersion
The modified barium sulfate was dispersed into the base formulation at different ratios using a combination of mechanical stirring and ultrasonic dispersion. Specifically, a certain amount of modified barium sulfate was weighed, added to deionized water, and ultrasonically dispersed for 10 min. The base formulation was then slowly added under stirring, and the mixture was stirred for another 30 min.
2. Optimal Modification Process and Performance Evaluation
(1) Optimal Modification Process
Through systematic research, the optimal process conditions were determined: Industrial-grade barium sulfate was sieved through a 200-mesh screen and dried at 60°C for 4h. Sodium dodecyl sulfate was used as the surface modifier at 1.5% of the barium sulfate weight, and the modification was performed at 60°C for 2h. In the dispersion process, the barium sulfate content was controlled at 15%–20%, the dispersion temperature at 60°C, the dispersion time at 15 min, and the system pH maintained at 8.0–8.5. A combination of mechanical stirring and ultrasonic dispersion was used.
Under these conditions, the resulting dispersion system exhibited the following characteristics: a uniform particle size distribution with a main particle size of 0.8–1.2 μm; good dispersant stability with no significant sedimentation within 7 days; and excellent coverage with a uniform and continuous film.
(2) Application Evaluation in Cosmetics
The prepared barium sulfate dispersion was evaluated in cosmetic formulations: Adding 15% of the modified barium sulfate dispersion to a foundation cream resulted in good coverage and a pleasant user experience, with good compatibility with the base matrix and no phase separation.
Adding 20% of the dispersion to a concealer formulation significantly improved coverage, maintained good stability, and provided a natural and long-lasting effect.
The application evaluation results demonstrate that the barium sulfate dispersion prepared using the optimized process exhibits excellent performance in cosmetic applications. ALPA specializes in ultrafine grinding and classification to maximize your product's value. Specializing in ultrafine grinding and classification of Barite.
The potential of montmorillonite in the field of new energy

Montmorillonite (MMT) is a layered silicate mineral. In its structure, the high-valence aluminum atoms in the aluminum-oxygen octahedra can be easily substituted by lower-valence atoms, resulting in a negative charge between the layers. To maintain the stability of the interlayer structure, montmorillonite adsorbs cations such as Na+, Ca2+, Mg2+, Al3+, and K+ from its surroundings. This characteristic gives montmorillonite strong adsorption and cation exchange capabilities. This unique structure and exchange capacity endow montmorillonite with significant potential for applications in the field of new energy technologies.
Lithium Battery Materials
(1) For Solid-State Electrolytes
Numerous studies have shown that montmorillonite (MMT), as a novel inorganic filler, can significantly improve the ionic conductivity and mechanical properties of solid polymer electrolytes (SPEs).
(2) Constructing Artificial SEI Layers
In artificial solid electrolyte interphase (SEI) films, layered montmorillonite-lithium (Li-MMT) imparts good mechanical properties to the SEI layer and provides Li+ transport channels, which helps suppress lithium dendrite growth. Benefiting from the fast Li+ channels in Li-MMT, a Li-LiFePO4 full cell assembled with a Li-MMT SEI layer exhibits superior rate performance, and maintains a high capacity retention of 90.6% after 400 cycles at 1C rate.
(3) Separator Optimization
MMT is used to optimize separators due to its excellent adsorption properties. Compared with commercial PE separators, the Li-MMT-modified separator has a higher Li+ concentration at the electrode/electrolyte interface, which reduces selective lithium deposition, weakens local current density, and suppresses dendrite growth.
(4) Optimizing Liquid Electrolytes
In lithium metal battery systems, compared to PEO electrolytes, montmorillonite exhibits stronger affinity with metallic lithium, with a zeta potential of +26 mV, which promotes the enrichment of lithium ions near the montmorillonite surface. With the adsorption and separation of lithium ions, the overpotential slightly increases to -57.7 mV, guiding lithium ions to migrate from montmorillonite and deposit on the copper current collector surface.
(5) Carrier Materials
Supercapacitors
Template Materials
Some natural minerals have specific morphologies, such as attapulgite, montmorillonite, halloysite, and diatomite, which are commonly used as templates to synthesize porous carbon materials with specific morphologies. Furthermore, conductive polymers with specific morphologies can be synthesized using the mineral template method. (2) Electrode Carrier Materials
To obtain active materials with specific morphologies, and simultaneously enhance the specific capacitance and improve the cycling stability, active materials can be loaded onto the surface of minerals such as montmorillonite and halloysite.
Methane Storage Materials
Currently, researchers are exploring the use of adsorption-based natural gas storage technology, which is economical, convenient, and safe, as an alternative to traditional compressed natural gas and liquefied natural gas technologies. Studies have shown that clay minerals play a positive role in the formation and development of shale gas reservoirs and possess gas storage capabilities.
Electrocatalytic Materials
Electrocatalysis is a type of catalysis that accelerates charge transfer reactions at the electrode/electrolyte interface, and has been widely used in fields such as electrochemical hydrogen evolution, oxygen evolution, and NOx reduction. Clay minerals such as montmorillonite have been widely used as carriers for photoelectrocatalytic electrode reaction components to prevent particle aggregation, improve the stability of sensitizer molecules, and enhance reaction selectivity.
Phase Change Thermal Energy Storage Materials
Phase change thermal energy storage materials (PCMs) are a new type of functional material that utilizes the heat absorption or release during phase change for thermal energy storage and release. Natural minerals play an important role in the field of phase change thermal energy storage. On one hand, natural minerals themselves are excellent inorganic phase change materials, and can be processed into high-performance phase change thermal energy storage materials after adding appropriate nucleating agents and thickeners. On the other hand, the porous structure of minerals can serve as an excellent carrier for phase change thermal energy storage materials.
Titanium dioxide powder coating modification

Surface modification of titanium dioxide powder (titanium white) is an important method to enhance its performance (such as dispersibility, weather resistance, gloss, and chemical stability). Common surface modification techniques can be broadly categorized into three types: inorganic coating, organic coating, and composite coating. The following is a detailed classification and brief introduction of these methods:
Inorganic Coating Modification
This method involves coating the surface of titanium dioxide particles with a layer of inorganic oxides or salts, forming a physical barrier to improve its chemical stability and optical properties.
1. Oxide Coating
Principle: Metal oxide hydrates (such as SiO₂, Al₂O₃, ZrO₂ etc.) are precipitated onto the surface of titanium dioxide particles, forming a uniform coating layer.
Process: Typically, a liquid phase deposition method is used, where metal salts (such as sodium silicate, aluminum sulfate) are added to the titanium dioxide slurry, and the pH is adjusted to precipitate the metal oxide hydrates onto the surface.
2. Composite Oxide Coating
Principle: Coating with two or more metal oxides (such as Al₂O₃-SiO₂, ZrO₂-SiO₂ etc.), combining the advantages of each component.
Features: Superior overall performance; for example, Al₂O₃-SiO₂ coating can simultaneously improve dispersibility and weather resistance, suitable for demanding automotive coatings and coil coatings.
3. Salt Coating
Principle: Using metal salts (such as phosphates, silicates, sulfates, etc.) to form an insoluble salt layer on the surface of titanium dioxide particles.
Organic Coating Modification
This method involves reacting organic compounds with the hydroxyl groups on the surface of titanium dioxide, forming an organic molecular layer to improve its compatibility with organic media. 1. Coupling Agent Coating
Principle: Utilizing the amphiphilic structure of coupling agents (such as silanes, titanates, and aluminates), one end binds to the hydroxyl groups on the titanium dioxide surface, while the other end reacts with the organic matrix (e.g., resin, polymer).
Functions:
Silane coupling agents: Improve the dispersibility of titanium dioxide in aqueous systems, commonly used in water-based coatings and inks.
Titanate/aluminate coupling agents: Enhance compatibility in oily systems such as plastics and rubber, reducing agglomeration during processing.
2. Surfactant Coating
Principle: Surfactants (such as fatty acids, sulfonates, and quaternary ammonium salts) adhere to the titanium dioxide surface through physical adsorption or chemical reaction, forming a charge layer or hydrophobic layer.
3. Polymer Coating
Principle: Grafting polymers (such as acrylates, epoxy resins, and siloxanes) onto the titanium dioxide surface through polymerization reactions.
Functions:
Form a thick coating layer, further protecting against chemical attack and improving weather resistance and mechanical properties.
Enhance compatibility with specific resins, suitable for high-performance composites and coatings.
4. Organosilicon Coating
Principle: Utilizing the low surface energy of polysiloxanes (silicone oil, silicone resin, etc.) to coat titanium dioxide particles.
Functions: Reduce surface tension, improve dispersibility and lubricity, commonly used in inks and cosmetics.
Composite Coating Modification
Combining the advantages of inorganic and organic coatings, a dual coating process (sequential or simultaneous) achieves complementary performance.
1. Inorganic-Organic Sequential Coating
Process: First, form a physical barrier with inorganic oxides (e.g., SiO₂), then perform organic modification with coupling agents or polymers.
Features: Balances weather resistance and compatibility, suitable for high-performance architectural coatings or automotive OEM paints. 2. Inorganic-Organic Simultaneous Coating
Process: Inorganic and organic coating agents are introduced simultaneously into the same reaction system to form a core-shell structure.
Features: The coating layer exhibits stronger adhesion and significantly improved performance, suitable for high-end applications (e.g., aerospace coatings, nanocomposites).
Other Special Coating Technologies
1. Nanoparticle Coating
Principle: Using nanoparticles (e.g., nano-SiO₂, nano-ZnO) for coating enhances UV protection and transparency, commonly used in sunscreen cosmetics and optical coatings.
2. Microencapsulation
Principle: Encapsulating titanium dioxide particles in polymeric microcapsules, releasing the titanium dioxide by controlling the capsule rupture conditions (e.g., temperature, pH), suitable for smart coatings and controlled-release systems.
The selection of different coating methods depends on the application (e.g., coatings, plastics, inks, cosmetics) and performance requirements (weather resistance, dispersibility, compatibility, etc.).