Titanium dioxide inorganic and organic coating modification technology

Rutile titanium dioxide is a semiconductor with a bandgap width of about 3.0eV. It has strong photocatalytic activity without surface modification, so that it can produce highly active oxygen free radicals under the radiation of solar ultraviolet rays. , this oxygen free radical can exert a strong oxidation ability, which will damage the medium around titanium dioxide and affect the service life of the product. Therefore, surface modification is an extremely important task in the production and processing of titanium dioxide.
Surface modification is the use of modifying additives to react with the surface of titanium dioxide, thereby changing the surface characteristics and improving the performance of the product. At present, the surface modification of titanium dioxide is roughly divided into two methods: inorganic coating and organic coating.
1. Titanium dioxide inorganic coating
Inorganic coating is to coat the surface of titanium dioxide particles with a single-layer or multi-layer inorganic thin film by means of sedimentation reaction, forming a barrier between the particles and the medium, so as to improve the performance of titanium dioxide. Inorganic surface modification of titanium dioxide is generally carried out by aluminum coating, silicon coating, zirconium coating and multiple mixed coating methods.
For silicon coating, the film formed under neutral and slightly acidic conditions is relatively "fluffy", while the film formed under alkaline conditions is relatively dense, generally through the hydrolysis of sodium silicate to generate silicon The micelles are then fixed on the surface of titanium dioxide through Ti-O-Si bonds, and at the same time, the formation of Si-O-Si bonds can also be used to ensure that the film is continuous and uniform.
For aluminum coating, the Ti-O-Al bond is formed through the reaction of OH-Al and the -OH group on the surface of titanium dioxide. The increase in the number of clusters facilitates the coating. At the same time, under high pH conditions, the directional growth rate of OH-Al occupies a dominant position relative to the sedimentation rate when the temperature is raised, and the film morphology changes from uniform and continuous sheet-like layers to relatively loose flocs.
Inorganic coating is specifically divided into two methods: dry coating and wet coating according to different processing methods.
(1) Titanium dioxide dry coating
In dry coating, metal halides are generally attached to the surface of titanium dioxide by air spraying, and after roasting and oxidation, hot steam is introduced to promote its hydrolysis to form a thin film coating on the particle surface.
(2) Titanium dioxide wet coating
Wet coating is mainly carried out in water medium, which is also subdivided into three types: boiling method, neutralization method and carbonization method.
2. Titanium dioxide organic coating
The development history of organic coating is shorter than that of inorganic coating, but it develops very rapidly due to the characteristics of small dosage (usually only 0.1% to 1% of the weight of the pigment) and large effect. There are three main methods of organic coating in the laboratory, namely high-speed dispersion wet method, vibration dispersion method and gas powder machine pulverization method. In the daily experiment process, we mainly adopt the high-speed dispersion wet method for processing.
Generally, in the process of organic coating, a part of the organic treatment agent is connected to the surface of the titanium dioxide by physical adsorption, and the other part reacts with the hydroxyl groups on the surface of the particles and then closely combines with the surface of the titanium dioxide. Dispersants, coupling agents, surfactants, etc. are used.
3. Composite coating with titanium dioxide
Since inorganic coating and organic coating have their own emphasis. Generally speaking, the main purpose of inorganic coating is to reduce the photocatalytic activity of titanium dioxide, improve its weather resistance, thereby increasing the service life of the product, while the main purpose of organic coating is to improve the dispersion ability of the product in various media and dispersion stability.
The two methods cannot replace each other, so in practical application operations, the operation mode of first inorganic coating and then organic modification is mostly used to modify the surface of titanium dioxide particles to achieve the purpose, that is, to use silicon, Soluble inorganic sources such as aluminum and zirconium (such as silicon dioxide, aluminum oxide, etc.) complete one or even multiple layers of inorganic coatings under their respective appropriate temperature and pH conditions to enhance their weather resistance. Then select a suitable bridging structure to connect fatty acid or aromatic acid groups with strong hydrophilicity to enhance its water dispersibility and dispersion stability.
Grinding of refractory raw materials

Crushing is an essential process in the refractory industry. The raw materials delivered to the factory range from powder to about 350mm, most of which are blocks over 25mm. The crushing process and raw material selection in the factory are the key to the production of high-quality products, which have a direct impact on the properties of the product. In addition, from the point of view of cost accounting, the power consumed by crushing and crushing equipment accounts for a large proportion. In order to save energy and reduce costs, attention must be paid to the crushed process.
The essence of the crushing process is related to the following factors, that is, overcoming the surface tension of the material's surface particles and overcoming the Coulomb attraction between the material's internal particles. Starting from the basic concept of the silicate physical and chemical dispersion system, it is not difficult to see that the particles of the crushed material are still very large when they are first crushed, so the surface and surface energy of the particles are small. , It is difficult to crush the material below 1μm (micron), the smaller the particle, the higher the surface energy, so when finely crushing, more energy will be consumed to overcome the surface energy. In addition, during fine grinding, due to the accelerated thermal movement of particles, the collision probability of particles increases, and coalescence and coagulation may also occur. Therefore, the crushing process must be organized correctly, and the crushing method and equipment should be selected according to the degree of dispersion of the final product.
The purpose of crushing:
(1) Crushing is an important operation link in the beneficiation process. When separating and enriching particles of the same component from raw ore aggregated by two or more different minerals, the raw ore should be crushed first in order to distinguish by type.
(2) In order to promote the interaction between the various phases, or evenly disperse the solid particles into the liquid, for example, prepare mud.
(3) Prepare various particle sizes according to process requirements. Increase the lattice defects and specific surface of the material, accelerate the physical and chemical reactions, and promote sintering.
The crushing methods can be roughly divided into the following four types: extrusion, impact, grinding and splitting. The function of various crushing machines is a combination of the above methods.
Crushing is divided into dry crushing and wet crushing. Wet crushing is mostly used in the production of ceramics or special refractory materials. Compared with dry crushing, it has the following advantages:
(1) The crushing ratio is large, and the particle size of the crushed material is small;
(2) The crushing efficiency is high, and the phenomenon of "powder wall" during dry crushing is not easy to occur (but when the particle size of the crushed product is less than 0.01mm, powder aggregation will also occur);
(3) The friction loss of equipment and grinding body is small;
(4) Good dust prevention, which is conducive to civilized production and process automation.
In addition, there are low-temperature crushing, dry crushing, and self-generating crushing based on the impact and friction of crushed materials, which are classified according to the crushing medium.
When crushing raw materials, the volume density and strength index of the material are of great significance to the selection of crushing equipment and the analysis of crushing efficiency.
The characteristics and application of zirconia powder

Zirconia ceramics is a new type of high-tech ceramics. In addition to its high strength, hardness, high temperature resistance, acid and alkali corrosion resistance and high chemical stability, it also has the characteristics of scratch resistance, no signal shielding, and excellent heat dissipation performance. , At the same time, it has strong machinability and good appearance effect, and is suitable for mass production.
1 High melting point
The melting point of zirconia is 2715°C. The higher melting point and chemical inertness make zirconia a good refractory material.
2 High hardness and good wear resistance
Zirconia ceramics have greater hardness and better wear resistance. From the specific data, the Mohs hardness of zirconia ceramics is about 8.5, which is very close to the Mohs hardness of sapphire 9, while the Mohs hardness of polycarbonate is only 3.0, the Mohs hardness of tempered glass is 5.5, and the Mohs hardness of aluminum-magnesium alloy The Mohs hardness of Corning glass is 6.0, and the Mohs hardness of Corning glass is 7.
3 Relatively high strength and toughness
Zirconia ceramics have high strength (up to 1500MPa). Although there is a big gap in toughness compared with some metals, compared with other ceramic materials, zirconia ceramics are considered to be the best in the "ceramic circle" (1-35MPa .m1/2).
4 Low thermal conductivity, low coefficient of expansion
The thermal conductivity of zirconia is the lowest among common ceramic materials (1.6-2.03W/(m.k)), and its thermal expansion coefficient is close to that of metal. Therefore, zirconia ceramics are suitable for structural ceramic materials, such as zirconia ceramic mobile phone appearance structural parts.
5 Good electrical performance
The dielectric constant of zirconia is 3 times that of sapphire, the signal is more sensitive, and it is more suitable for fingerprint recognition patches, etc. From the perspective of shielding effectiveness, zirconia ceramics, as a non-metallic material, have no shielding effect on electromagnetic signals, and will not affect the internal antenna layout at all, and can be easily integrated to adapt to the 5G era.
Zirconia ceramics are widely used in modern industry and life. Let's briefly introduce its main applications.
1 Mobile phones and other 3C electronics fields
Zirconia ceramics have no signal shielding, are resistant to falling, wear and folding, and at the same time have a warm and jade-like appearance and a good hand feel. They are widely used in 3C electronics such as mobile phones. Mainly used as mobile phone backplane and other mobile phone structural parts.
2 Smart wear field
Compared with metal, zirconia ceramics have better wear resistance, smooth surface, good texture, and no oxidation. Well-known brands such as the famous Swiss "Radar" brand, Apple, and Chanel have launched high-end ceramic watches.
3 Optical communication field
At present, ceramic ferrules and sleeves are widely used in optical fiber connector connectors. The ceramic ferrule made of high-strength and high-toughness ceramics can not only meet the high-precision requirements, but also have a long service life and very low insertion loss and return loss.
4 Biomedical field
Due to high strength, high toughness, corrosion resistance, wear resistance and good biocompatibility, zirconia ceramic materials are most commonly used in the field of biomedicine as dental restoration materials and surgical knives.
5 Automotive field
The thermal conductivity of zirconia ceramics is small, and the thermal expansion coefficient is relatively large, so the components used to make the engine combustion chamber have good heat insulation, and at the same time, they are closer to metal materials in terms of thermal expansion. It can be used as cylinder head bottom plate, cylinder liner, piston crown, valve seat ring, etc. However, due to the harsh working conditions of the engine, the strength of ceramic components changes greatly at high temperatures, so there is still a long way to go before commercial application.
6 Jewelry field
High-precision ceramics and precious metal alloy powder are mixed and fired, and finally integrated into jewelry design after several precise and strict procedures and multiple machine polishing. This ceramic is not only light and wear-resistant, but also has anti-sensitivity properties and is comfortable to wear.
7 Daily life
Ceramics have the characteristics of high temperature resistance, corrosion resistance, oxidation resistance, high strength, wear resistance, and natural antibacterial properties, and can be used as porcelain bowls and spoons, vases, ceramic knives, etc.
8 Other fields
Zirconia ceramics have good mechanical properties, and are wear-resistant and corrosion-resistant. They can be used as ceramic bearings and can also be made into ceramic knives.
Preparation and Present Situation of Ultrafine Non-metallic Mineral Powder
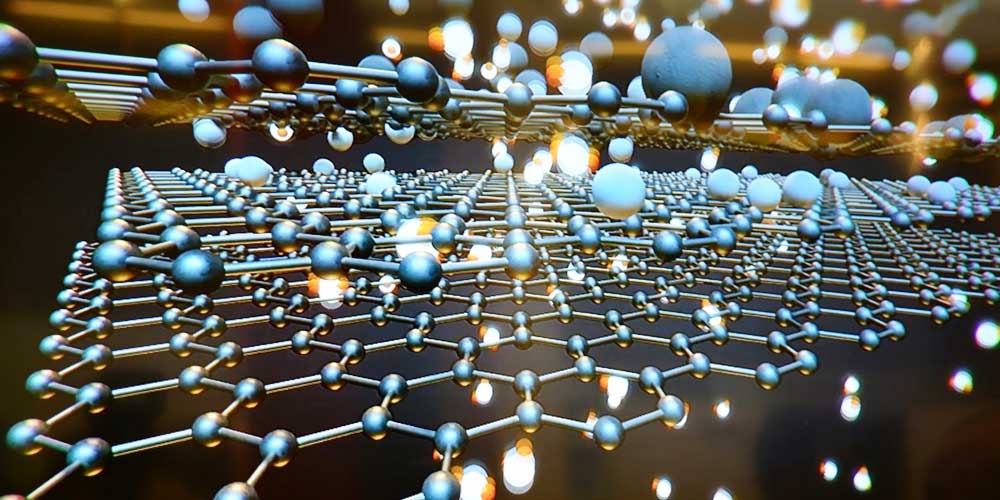
With the application of non-metallic mineral resources in various fields of economy and society, the development of non-metallic mineral resources has been significantly strengthened. Since these non-metallic minerals are used in many fields, there is a form of powder utilization, which makes non-metallic mineral powder in the industry. Processing technology puts forward higher requirements, such as ultra-fine.
Ultrafine powder refers to a series of ultrafine materials with particle sizes ranging from micrometers to nanometers. At present, the wide application of non-metallic mineral powders in modern high-tech new materials is based on their unique functions. The function of most non-metallic minerals depends on the particle size, distribution and particle shape. Such as reinforcement or reinforcement in polymer-based composite materials, strength and toughness of ceramic materials, covering ratio, coloring power as pigments for papermaking and coatings, and electrical, magnetic, optical, wave-absorbing and shielding properties of powders , catalysis, adsorption, rheology, antibacterial, decolorization, bonding, etc. are all related to its particle size, particle size distribution and particle shape.
Due to the ultrafine powder has excellent physical and chemical properties such as large specific surface area, high surface activity, fast chemical reaction speed, low sintering temperature, high sintered body strength, good filling and reinforcing performance, and high covering rate. Many application fields require fine particle size (micron or submicron) of non-metallic mineral raw materials (materials).
At present, in the processing of ultrafine non-metallic ore powder, physical method is the main preparation method. And generally speaking, the process of making raw materials into ultrafine powder is mainly divided into two steps: crushing and classification. The material first enters the ultra-fine crushing equipment for crushing. Because the structure of each particle is different, the energy required for crushing is different, and the force received in the crushing equipment is not equal, so the shape and size of the fine particles after crushing are not the same. , only part of the particles meet the particle size requirements. In the actual production process, the particles are often fully crushed by prolonging the crushing time to meet the particle size standard, which not only increases energy consumption, but may also lead to excessive crushing. Therefore, it is necessary to separate the particles with the required particle size in time, so the ultrafine classification technology also plays an important role in the preparation process of ultrafine powder.
At present, the commonly used ultrafine grinding equipment mainly includes impact mill, stirring mill, jet mill and vibrating mill. Regardless of how the powder industry develops, the main means of obtaining ultra-fine non-metallic mineral powders is still mechanical pulverization.
The classification of ultrafine powder is based on the fact that particles of different particle sizes are subjected to centrifugal force, gravity, inertial force, etc. in the medium, resulting in different motion trajectories, so as to realize the separation of particles of different particle sizes and enter their respective collection devices.
According to the different media used, the ultra-fine grade is generally divided into two types: dry type and wet type. Wet classification uses liquid as the dispersion medium, with high classification accuracy and good uniformity. However, there are a series of follow-up operational problems such as drying and wastewater treatment in wet classification, which limits its development.
At present, the classification equipment widely used in industrial production is the turbine air classifier, which can be divided into vertical wheel type and horizontal wheel type according to the installation form of the classifying wheel.
In the course of years of exploration and practice, non-metallic ore powder ultra-fine processing technology is becoming more and more mature, and there are more and more technical processes and equipment on the market. In order to improve production capacity and efficiency, relevant enterprises are carrying out non-metallic ore powder processing. In the process, combined with its own production reality and needs, make a comprehensive selection of technologies, processes and equipment, and strengthen the control of relevant parameters and process adjustments in the processing process.
Application of lithium minerals in the production of high-grade glass and ceramics

With the advent of new energy vehicles, lithium batteries have become the focus of attention and the subject of scientific research. Lithium-containing minerals not only have great potential in the field of new energy, but also have important functions and play a special role in the high-grade glass industry. Both spodumene and petalite are lithium-containing minerals and are raw materials for extracting lithium. The two are often produced in granite pegmatites and become paragenetic minerals. Because of its special physical and chemical properties, it is widely used in the production of high-grade glass and ceramics.
1. Glassware
In the production of glassware, although lithium oxide is not an important part of the glass composition, it has excellent melting ability, which can reduce the melting temperature, prolong the service life of the furnace, improve the melting efficiency, and thus improve product quality. Adding spodumene concentrate can be used to produce high-grade glassware for packaging of cosmetics. Low-grade glass-grade spodumene has also been gradually accepted by the market.
2. Tableware
In the production of containers, the Fe2O3 content of tableware is significantly lower than that of similar products. The use of spodumene with high lithium oxide content and low iron content can ensure that the product meets the specified color requirements. In addition, high-quality spodumene can not only lower the melting point, but also reduce the viscosity of the melt. Therefore, the formability is good, and the production efficiency will be significantly improved.
3. Fiberglass
The use of lithium oxide in glass fiber production can not only reduce the damage of fluorine to the environment, but also have the same effect as in the production of glassware, such as lowering the melting point and improving the melting effect, thereby improving production quality. The viscosity of the melt is low, easy to operate, low working temperature and long service life of the equipment.
4. TV display screen
Lithium oxide extracted from spodumene concentrate or petalite is the main component of monochrome televisions. The combination of lithium oxide and barium reduces the radiation transmitted through the panel, improving the molding characteristics and surface finish of the display. In the application of color TV, since the use of lead is gradually banned, it is replaced by lithium oxide. Zirconia and barium are increasingly used in formulations, while lithium oxide is used as a flux.
5. High temperature ceramic products
In the established ceramic industry, lithium is an important part of the formulation. Spodumene as a low expansion rate filler contributes to the formation of the low expansion rate lithium aluminosilicate phase. Add a large amount of spodumene, and choose an appropriate calcination temperature, the following reactions occur:
Li2O.Al2O3.aSiO2+SiO2= Li2O.Al2O3.8SiO2
(spodumene) + (silicon oxide) = (β-spodumene solid solution)
Free silica is assimilated in β-spodumene solid solution, exhibiting almost negligible thermal expansion. Therefore, the product has thermal shock resistance.
6. Glaze
Lithium oxide can be used to reduce the viscosity of the melt and improve the fluidity of the coating. It can also reduce firing time and firing temperature.
7. Fully vitrified ceramics
Spodumene plus feldspar flux can reduce the firing temperature of general sanitary ware by 30-40°C. The Italians added spodumene to the ultra-white ceramic body to reduce the shrinkage effect and thus improve production efficiency. The low porosity green body with spodumene added ensures minimum dust absorption while increasing combustion efficiency.
With the wide application of lithium oxide in ceramics, glass fiber, flat glass and color TV, etc., it has gradually expanded to the metallurgical industry. Lithium oxide can be used to change the viscosity of slag, improve metal recovery and reduce the possibility of slag in the metal.
Nano calcium carbonate surface modification effect

The evaluation of modification effect is an essential link in the modification process. Some conjectures can be verified by some detection methods, and the modification process can be adjusted and optimized by analyzing its influencing factors to improve the performance of nano-calcium carbonate.
There are mainly two traditional evaluation methods, one is to directly detect and evaluate the modified sample, and the other is to make the modified sample into a composite material to investigate the performance improvement effect of the composite material due to modification. In comparison, direct evaluation is fast and efficient.
1. Activation index and oil absorption value
Activation index and oil absorption value are commonly used evaluation indicators for the modification effect of nano-calcium carbonate. The activation index can be used to evaluate the hydrophobic effect of nano-calcium carbonate after surface modification, and the oil absorption value refers to the oil consumption of nano-calcium carbonate in application. Generally speaking, the higher the activation index and the lower the oil absorption value, the better the modification effect.
2. Hydrophobicity
Hydrophobicity is an important evaluation index of nano-calcium carbonate, and it is also a research hotspot in the modification of nano-calcium carbonate. The static contact angle can be used to characterize the hydrophobicity of nano-calcium carbonate. The type of modifier has a significant impact on the hydrophobicity of modified nano-calcium carbonate. Stearic acid, silane coupling agent, oleic acid, titanate coupling agent, etc. are commonly used hydrophobic modifiers. During the surface modification process, these modifiers are gradually attached to the surface of the particles, thereby reducing the surface energy of the nano-calcium carbonate particles.
3. Coating amount and coating rate
By detecting the coating amount and coating rate, the coating situation of nano-calcium carbonate can be understood, which is of great help to the study of the modification mechanism and the evaluation of the modification effect. Usually, according to the decomposition temperature or volatilization temperature of different substances, the modified nano-calcium carbonate can be subjected to thermogravimetric analysis to obtain the coating amount of the modifier, and then the coating ratio can be obtained.
In addition, some researchers have constructed a corresponding coating model through the study of the modifier mechanism, thereby calculating the theoretical coating amount or coating rate, and understanding the coating situation by comparing it with the actual coating amount or coating rate. , and also provides a practical basis for the study of the modification mechanism.
4. Particle size and shape
The particle size and morphology of nano-calcium carbonate mainly depend on its preparation process. Therefore, in the process of in-situ modification, process conditions such as liquid phase concentration, stirring rate, temperature, and the type and concentration of modifiers will affect the nano-calcium carbonate. By controlling the nucleation, crystallization and growth of these factors, nano-calcium carbonate with different shapes and sizes can be prepared.
5. Whiteness
For coatings, papermaking, rubber, plastics and other industries, whiteness is an important indicator for evaluating nano calcium carbonate. The whiteness of modified nano-calcium carbonate is not only related to the choice of modifier, but also related to moisture, drying temperature and drying time. Generally, the longer the drying time, the higher the temperature and the less moisture, the The higher the whiteness.
6. Dispersion
Nano-calcium carbonate can be widely used as a filler in rubber, plastic, paper and other industries. Therefore, the dispersion of nano-calcium carbonate in the organism is also an important evaluation index. By scanning the filled organism with an electron microscope, it can be visually observed Distribution of nano calcium carbonate. In addition to the performance and modification effect of nano-calcium carbonate itself, its filling amount is also an important factor affecting the dispersion.
Organic Modification Method of Clay Minerals

Compared with other adsorbents, clay minerals are often used as natural adsorbents due to their low cost, large specific surface area, and high cation exchange capacity.
In recent years, people use natural clay minerals such as kaolinite, montmorillonite, illite and bentonite to remove organic pollutants and anion pollutants in water. However, studies have shown that natural clay minerals have a certain adsorption capacity for anionic pollutants, but their adsorption capacity for organic pollutants is weak. This is because there are many hydrophilic inorganic cations on the surface of clay minerals, making the surface of clay minerals hydrophilic in a wet state, and it is difficult to directly adsorb hydrophobic organic pollutants.
By modifying natural clay minerals with surfactants, polymers, and silane coupling agents, the surface of clay minerals can be transformed from hydrophilic to hydrophobic, and an organoclay adsorbent with low cost and strong adsorption performance can be obtained. It can effectively improve the adsorption of clay minerals to hydrophobic organic pollutants.
1. Surfactant
Surfactant molecules are composed of two groups with completely different properties, namely hydrophilic group and hydrophobic group. According to the dissociation of hydrophilic groups in aqueous solution, surfactants can be divided into cationic surfactants, anionic surfactants and nonionic surfactants. And because of its environmental friendliness and low toxicity, it is often used as a clay modifier.
(1) Cationic surfactant
The mechanism of using cationic surfactants to modify clay minerals is usually an ion exchange reaction, that is, organic cations in cationic surfactants replace inorganic cations (such as Na+, Ca2+, etc.) between clay mineral layers.
(2) Anionic surfactants
The hydrophilic groups of anionic surfactants are negatively charged groups, and there are also negatively charged groups on the surface of clay minerals, so that anionic surfactants cannot be adsorbed on the surface of clay minerals by electrostatic attraction. At present, the modification mechanisms of anionic surfactants on clay minerals are mainly hydrophobic bonding and hydrogen bond formation.
(3) Cationic and anionic composite surfactants
(4) Gemini surfactants
Gemini surfactants (dimer surfactants) are composed of two hydrophobic alkyl carbon chains and hydrophilic groups, linking groups and counter-ionic groups. Compared with traditional alkyl quaternary ammonium cationic surfactants, clay minerals modified by gemini surfactants usually have higher adsorption capacity and lower modifier release, so they are widely used in the field of sewage removal.
(5) Non-ionic surfactants
Nonionic surfactants do not dissociate in water, and their hydrophilic groups are usually ester groups, carboxyl groups, and hydroxyl groups, which can interact with hydroxyl groups on the surface of clay minerals to generate hydrogen bonds and adsorb on the surface of clay minerals.
In addition, it has been reported that organoclay minerals modified by nonionic surfactants have larger interlayer spacing and higher chemical stability than organoclay minerals modified by cationic surfactants, and have better application prospect.
2. Polymer
Polymers can modify clay minerals through physical adsorption, ion exchange and chemical grafting, and improve the adsorption performance of clay minerals.
The physical adsorption modification method refers to that the polymer is adsorbed on the surface of the clay mineral due to its own charged or functional groups forming hydrogen bonds with the hydroxyl groups on the surface of the clay mineral, and changes the physical and chemical properties of the surface. The advantage of physical adsorption is that it does not change the structure of clay minerals. The disadvantage is that the force between the polymer and the clay mineral surface is relatively weak, and it is easily disturbed by factors such as temperature and pH value.
The chemical grafting of polymers to the surface of clay minerals belongs to chemical adsorption, and the condensation of polymers and reactive groups of clay minerals makes the polymers bonded to the surface of clay minerals. Clay minerals modified by chemical adsorption are more stable than those modified by physical adsorption.
3. Silane coupling agent
Silane coupling agents, also known as organosilanes, are composed of non-hydrolyzable groups, short-chain alkylene groups, and hydrolyzable groups. Silane coupling agents modify clay minerals, usually by hydrolyzing the hydrolyzable groups of silane into hydroxyl groups and then condensing with the hydroxyl groups on the surface of clay minerals to form stable Si-O-Si or Si-O-Al covalent bonds and adsorbed on the clay. mineral surface.
Four major development trends of calcium carbonate technology for papermaking

As an important paper-making filler and coating pigment, calcium carbonate has shown its unique advantages and has the potential to continue to flourish. As the paper industry has stricter requirements on product quality and more diversified product types, surface modification, nanotechnology, specialization and the development of new calcium carbonate products will become a new direction for the development of calcium carbonate product technology.
Calcium carbonate is an inorganic substance, the surface of the particles is polar, hydrophilic and oleophobic, and has agglomeration, poor compatibility with organic polymers, uneven dispersion in polymer base materials, low binding force, and easy to produce interfaces Defects lead to unstable product quality. Calcium carbonate without surface modification as a paper-making filler has disadvantages such as poor compatibility and binding force with pulp fibers, low retention rate in paper, and reduced mechanical strength of paper. Therefore, calcium carbonate needs to be surface modified in order to be better used in the paper industry.
The surface modification process of calcium carbonate mainly includes dry modification process, wet modification process and in-situ modification process. Generally, heavy calcium carbonate prepared by dry grinding adopts dry modification process, and heavy calcium prepared by wet grinding adopts wet modification process. Light calcium carbonate is prepared by chemical method, generally using in-situ modification process. Commonly used modifiers for surface modification of calcium carbonate for papermaking mainly include coupling agents, polymers, and inorganic substances.
2. Nanoization
After adding nano-calcium carbonate fillers in the papermaking process, the paper has the following characteristics: it can slow down the aging of the paper, so that the paper can be stored for a longer time; it can make the paper absorb a certain amount of ultraviolet rays; it makes the paper not easy to yellow or fade Brittle, and has good isolation properties, etc.
Nano-calcium carbonate is used as a paper-making coating pigment, which is beneficial to improve the gloss, whiteness and coating hue of the coated paper; it can ensure the purity of the white pigment color; it is beneficial to improve the opacity, gloss and printing gloss of the paper, etc. Optical properties; can change the rheological properties of the coating preparation solution; realize the functionalization of the coating paper, such as insulation, conductivity, antibacterial properties, etc.
As a paper-making filler, nano-calcium carbonate is generally used in the production of special paper products, such as diapers, sanitary napkins, color-jet printing paper, paper towels and breathable films.
3. Specialization
Different papers have different properties and require different calcium carbonate properties. In order to improve the economic value, the corresponding calcium carbonate product can be developed for a certain kind of paper, so that it can reduce the production cost while meeting the usage requirements.
High-grade cigarette paper requires that the light calcium carbonate used as a filler should have a relatively complete spindle-shaped crystal form, with uniform and orderly crystal grains; its particle size is mainly distributed around 1-2 μm, and there are no large-size particles (>5 μm); and Good dispersion and bonding performance in pulp.
4. Develop new products of calcium carbonate
(1) mixed calcium carbonate
Mixed calcium carbonate (HCC) is to use ionic polymer to prepare the mixture of ground calcium carbonate and calcium oxide into pre-agglomerates, and then treat the pre-agglomerates with carbon dioxide to form new calcium carbonate between GCC and finally form carbonic acid calcium products. The post-mixed calcium carbonate preparation process is roughly the same as the HCC preparation process, except that the first aggregate is formed only from ground calcium carbonate, and after the ground calcium carbonate pre-agglomerate is prepared, the same amount of calcium oxide as the HCC process is added, and then carbon dioxide is injected. New calcium carbonate is formed on the outside of the GCC first aggregate, and the final calcium carbonate product is post-mixed calcium carbonate (PostHCC or pHCC).
(2) Calcium carbonate whiskers
Calcium carbonate whiskers belong to the aragonite calcium carbonate crystal structure, have high elastic modulus, heat resistance, wear resistance and heat insulation and other good properties, and have the whisker material with large aspect ratio, short fiber and small diameter ( Micron level) and high strength characteristics. It is widely used in the fields of papermaking, cement materials, building materials, coatings and automobile manufacturing materials.
Surface modification method of silicon micro powder
![]()
In the process of application, silicon micro powder is mainly composed of functional fillers with organic polymer polymers, thereby improving the overall performance of composite materials. Silicon micro powder itself is a substance of polarity and hydrophilicity. It is different from the interface attributes of the matrix matrix of the polymer polymer and is poorly compatible. It is often difficult to disperse in the base material. Therefore, the surface modification of silicon micro powder is usually required. Depending on the needs of the application, the physical and chemical properties of the silicon micr -powder surface are changed, thereby improving the compatibility of its organic polymer materials, and meeting the decentralization and liquidity needs of polymer materials.
Silicon micro -powder ingredients quality, modification process, surface modification method and modified agent, modifier dosage, modified process conditions (modifier temperature, time, pH, and mixing speed) and other factors all affect the surface modification effect of silicon microfanten surface. The surface modification method and modifier are the main factor affecting the modified effect.
1. Silicon microfin raw material quality
The types, particle size, surface area, and surface -oriented group of silicon powder directly affect the combination of its surface modifiers. Different types of silicon micro -powder modification effects are also different. Among them, spherical silicon micro powder has good liquidity. It is easy to combine with the modifier during the modification process. And the performance of density, hardness, and dielectric constant is significantly better than that of corner silicon microfim.
Generally, the smaller the particle size of the silicon microfanten, the larger the surface area, the more the number of active sites on the surface, and the increase in the amount of the modifier. In addition, in the process of application of silicon microfimmes of different granularity, it also has a certain impact on the performance of downstream products. For example, in the process of mixed with resin mixing with resin, the particle size distribution should be strictly controlled. It should not be too large or too small. The size of the size is too large. Essence
2. Surface modification method and modified agent
At present, the surface modification method of silicon micro powder is mainly organic modification, inorganic modification, and mechanical chemical modification. The most commonly used method is organic modification. When a single modification effect is poor
(1) Organic modification
Organic modification is a method of physical adsorption, chemical adsorption, and chemical reactions on the surface of silicon microfilling on the surface of silicon micro -powder to change the surface properties of silicon microfan. At present, the most commonly used organic modified agent is a sibidine coupling agent, which mainly includes amino, epoxy, ethylene, sulfur, and other types. The modification effect is usually good, but the price is expensive. Some researchers use aluminate, titanate, and hard fatty acid to make silicon microfimmeter with relatively low prices, but the modification effect is often not as good as the silicane coupling agent. Two or more surfactants are compounded to the silicon microfimmeter, and the modified effect is often more ideal than that of a single modifier.
(2) Inorganic modification
Inorganic modification refers to a new function of materials to give materials on the surface of silicon microfimy or composite metal, inorganic oxides, hydroxide, etc. For example, Oyama and others use precipitation methods to cover AL (OH) 3 on the surface of the SIO2, and then use the SiO2 after the polyethylene -based phenylphenylene wrapping, which can meet some special application needs.
(3) Mechanical chemical modification
Mechanical chemical modification refers to the first use of ultra -fine crushing and other strong mechanical power to activate the surface of the powder particles to increase the active point or active group on the surface of the silicon microfan, and then combine the modified agent to achieve the compound modification of silicon microfan.
Application of jet pulverization equipment in the production of titanium dioxide

1. Principle of jet milling
Jet milling equipment includes jet mill, jet mill or fluid energy mill, which uses the energy of high-speed airflow or superheated steam to make particles impact, collide, and rub against each other to achieve ultrafine pulverization or depolymerization. The general principle of jet milling: Dry and oil-free compressed air or superheated steam is accelerated into a supersonic airflow through the Laval nozzle, and the high-speed jet ejected drives the material to move at a high speed, causing the particles to collide and rub against each other to be crushed. The crushed materials arrive at the classification area with the airflow, and the materials that meet the fineness requirements are finally collected by the classifier, and the materials that do not meet the requirements are returned to the crushing chamber to continue crushing.
2. Classification of jet milling equipment
There are mainly several types of jet mills used in my country's industry: flat jet mill, fluidized bed jet jet mill, circulating tube jet mill, counter jet jet mill, and target jet mill. Among these types of jet mills, flat jet mills, fluidized bed jet mills, and circulating tube jet mills are widely used.
2.1 Counter jet jet mill
After the material enters the crushing chamber through the screw feeder, the impact energy of the high-speed airflow is sprayed out by several relatively set nozzles, and the rapid expansion of the airflow forms the collision and friction generated by the suspension and boiling of the fluidized bed to crush the material. Coarse and fine mixed powder is driven by the negative pressure airflow through the turbine classification device installed on the top. The fine powder is forced to pass through the classification device and is collected by the cyclone collector and bag filter. The coarse powder is thrown away by gravity and the centrifugal force generated by the high-speed rotating classification device. It goes to the four walls and settles back to the crushing chamber to continue crushing.
2.2 Flat jet mill
The high-pressure airflow as the crushing kinetic energy enters the pressure-stabilized air storage bag on the periphery of the crushing chamber as an air distribution station. The airflow is accelerated into a supersonic airflow through the Laval nozzle and then enters the crushing chamber, and the material is accelerated into the crushing chamber through the Venturi nozzle. Perform simultaneous crushing. Since the Laval nozzle and the crushing chamber are installed at an acute angle, the high-speed jet stream drives the material to circulate in the crushing chamber, and the particles collide, collide, and rub against each other as well as with the wall of the fixed target plate to be crushed. Driven by the centripetal airflow, the fine particles are introduced into the central outlet pipe of the pulverizer and enter the cyclone separator for collection, while the coarse powder is thrown to the surrounding wall of the pulverization chamber under the action of centrifugal force for circular motion and continues pulverization.
2.3 Circulating tube jet mill
The raw material is fed into the crushing chamber through the Venturi nozzle, and the high-pressure air is sprayed into the runway-shaped circulating tubular crushing chamber with unequal diameter and variable curvature through a group of nozzles, accelerating the particles to collide, collide, rub and crush each other. At the same time, the swirling flow also drives the crushed particles upwards into the classification area along the pipeline, and the dense material flow is shunted under the action of the centrifugal force field in the classification area, and the fine particles are discharged after being classified by the louver type inertial classifier in the inner layer. Coarse particles return along the downpipe in the outer layer and continue to be pulverized in a circular manner.
2.4 Fluidized bed jet mill
Jet mill (fluidized bed jet mill) is the compressed air that is accelerated by the Laval nozzle into a supersonic airflow and then injected into the crushing area to make the material fluidized (the airflow expands to form a fluidized bed that suspends and boils and collides with each other). Therefore every particle has the same motion state. In the pulverization zone, the accelerated particles collide with each other and pulverize at the junction of each nozzle. The crushed material is conveyed to the classification area by the updraft, and the fine powder meeting the particle size requirement is screened out by the classifying wheels arranged horizontally, and the coarse powder not meeting the particle size requirement is returned to the crushing area for further crushing. Qualified fine powder enters the high-efficiency cyclone separator with the airflow to be collected, and the dusty gas is filtered and purified by the dust collector and then discharged into the atmosphere.


