The preparation method of vaterite calcium carbonate

There are three common crystal forms of calcium carbonate: aragonite, vaterite, and calcite. From the perspective of thermodynamic stability, the calcite type is the most thermodynamically stable crystal form and exists widely in nature; while the vaterite type is the most unstable, in a metastable state, and only exists in some fish in nature. Otolith organs, ascidian spicules, crustacean tissues.
There are two main ways to generate vaterite calcium carbonate, namely dissolution recrystallization and solid-solid phase direct transformation. At present, it is believed that the dissolution and recrystallization route is the main way to generate vaterite-type calcium carbonate, that is, amorphous calcium carbonate is generated as the initial phase in the solution. However, the solubility of vaterite-type calcium carbonate is relatively high, and dissolution and subsequent nucleation and growth of calcite-type calcium carbonate occur. Such a process occurs continuously, making vaterite-type calcium carbonate gradually transform into calcite-type calcium carbonate.
Starting from the formation route and mechanism, high-purity vaterite-type calcium carbonate is mainly prepared by inhibiting the dissolution and recrystallization process. At present, common preparation methods can be divided into three types: carbonization method, metathesis method and thermal decomposition method according to the principles involved in the synthesis process.
1. Carbonization
The carbonization method uses an alkaline solution containing soluble calcium salts as a calcium source, and prepares vaterite-type calcium carbonate by introducing CO2 gas into the solution and controlling the process conditions. The calcium source is mainly divided into two kinds of calcium hydroxide aqueous solution and calcium chloride alkaline solution. Therefore, two major systems prepared by carbonization method are also determined: Ca(OH)2-H2O-CO2 reaction system and CaCl2-NH3·H2O -CO2 reaction system. A large number of studies have shown that both systems can produce vaterite calcium carbonate well.
However, the carbonization method has the advantages of low cost and simple process equipment, and is currently the main industrial production method for preparing various types of calcium carbonate products at home and abroad. At the same time, researchers at home and abroad have increased the mass transfer rate and dispersion of CO2 gas in the solution by using devices such as gas dispersers, and improved the efficiency and yield of vaterite-type calcium carbonate. Therefore, vaterite-type carbonic acid is prepared by carbonization. Calcium has great application prospects.
2. Double decomposition method
The double decomposition method refers to mixing the calcium salt solution and the carbonate solution under certain conditions to generate a double decomposition reaction, and at the same time adding a crystal form regulator and controlling the reaction temperature, concentration and other factors to control the preparation of vaterite calcium carbonate. In general, during preparation, one solution can be quickly mixed into another solution for the reaction, or one solution can be introduced into the other solution by controlling the addition rate for the reaction, and stirring is required at the same time. Promote the metathesis reaction.
3. Thermal decomposition method
Thermal decomposition method is a new method for preparing vaterite calcium carbonate, mainly refers to the preparation of vaterite calcium carbonate by thermal decomposition of calcium bicarbonate and controlling conditions. Usually, the purpose of preparing vaterite-type calcium carbonate is achieved by controlling the decomposition temperature, decomposition time, stirring mode and additives by using a saturated aqueous solution of calcium bicarbonate.
The preparation principle of the thermal decomposition method is simple, the process is short, and the equipment requirements are low, but the purity of the product vaterite calcium carbonate is low, the decomposition time is long, and the decomposition reaction is difficult to control; at the same time, the temperature required in the production process is high and energy consumption is high. large and difficult to apply in practice. There are few domestic and foreign studies on this method, and a lot of work still needs to be done in theory and practice.
Effect of Modified Mica Filler on Anticorrosion Properties of UV Cured Coatings

As an important part of the anti-corrosion coating, the anti-corrosion filler is one of the decisive factors affecting the corrosion resistance of the coating. Divided from the mechanism of action, anti-corrosion fillers mainly include active anti-corrosion fillers, sacrificial anti-corrosion fillers and shielding anti-corrosion fillers. Among them, shielding anti-corrosion fillers such as clay, boron nitride, mica, etc., these fillers will not react with the corrosive medium, and their unique lamellar structure can form a multi-layer dense barrier layer, effectively preventing the penetration of the corrosive medium and providing a good coating for the coating. Anti-corrosion effect, so it has been widely used.
As a silicate mineral, mica has excellent acid and alkali resistance, heat resistance and chemical stability. The natural ultra-fine crystal granular and lamellar structure enables mica to be easily processed into scaly ultra-fine powder. The thickness of the lamella can be controlled below 1 μm, which is difficult to achieve with artificial synthetic flakes such as glass flakes and stainless steel flakes. It is an ideal anti-corrosion filler, so it has received extensive attention.
The influence of the size effect of mica filler on the diffusion behavior of water in epoxy coatings was explored by mass method and electrochemical impedance method, and it was proved that suitable mica size can effectively block the penetration of water molecules; Meng et al. After modification, a mica-modified epoxy resin coating was prepared, and the failure behavior of the coating under the action of marine alternating hydrostatic pressure (AHP) was explored. It was found that surface modification could effectively improve the dispersion of mica in the coating.
Mica is used as anti-corrosion filler, anionic dispersant BYK-111 composed of non-polar negatively charged hydrocarbon chain part and polar hydrophilic group, and non-polar positively charged alkoxyammonium salt compound are used Different types of wetting and dispersing agents, such as BYK-180, phosphate ester salt type polymer BYK-145, and high molecular weight block copolymer BYK-168 containing pigment affinity groups, modify the surface of mica. And control the amount of mica added to explore the effect of mica filler on the curing rate, curing degree, adhesion, hardness and other properties and anti-corrosion performance of light-cured coatings. The results show that:
(1) The addition of mica filler has little effect on the degree of light curing and curing rate; the addition of mica can improve the adhesion of the coating, from level 1 to level 0, the impact on the hardness of the coating depends on the amount of mica in the coating. degree of dispersion;
(2) The unmodified mica has poor dispersibility in the coating and is easy to agglomerate. Not only can it not improve the corrosion resistance of the coating, but it will lead to a large number of defects in the coating and accelerate the occurrence of corrosion; different types of wetting and dispersing are used. The surface modification of mica by the agent can greatly improve the dispersibility of mica in the coating, thereby improving the anti-corrosion performance of the constructed light-cured coating.
(3) Amphiphilic high molecular weight block copolymer BYK-168 wetting and dispersing agent (high molecular weight block copolymer containing pigment affinity group) has the best modification effect on mica filler, 30% The addition amount of modified mica is the optimal addition amount, and the prepared photocurable coating is resistant to neutral salt spray for more than 1000h.
Preparation of Activated Calcium Carbonate from Calcium-Based Waste Residue and Its Effect on PVC Properties

As the earliest industrialized thermoplastic, PVC has good comprehensive mechanical properties, excellent flame retardant and corrosion resistance, but is brittle during processing, and must be modified after a series of impact resistance and toughening before use. Adding an appropriate amount of calcium carbonate in the PVC modification process improves the toughness, rigidity, strength, heat resistance and other indicators of the product, and at the same time, the cost of PVC application is greatly reduced.
As a kind of inorganic filler, in the process of PVC modification, the direct addition of untreated calcium carbonate will cause regional agglomeration. The product has poor dispersibility in the PVC system and weak interface affinity, which cannot achieve the expected improvement. Therefore, calcium carbonate must be organically modified to eliminate the surface potential energy of calcium carbonate, increase the wettability, dispersibility and hydrophobicity and lipophilicity of calcium carbonate in the PVC matrix, and improve the modification effect of calcium carbonate on PVC.
Calcium carbonate was prepared by using industrial waste residue and waste gas as raw materials, and it was modified. The influence of modified calcium carbonate on the properties of PVC was investigated. The results showed that:
(1) Using calcium-based waste residue (main component CaO) and CO2 produced in industrial production as raw materials, the best production process for preparing calcium carbonate through digestion, emulsion removal, carbonization, etc. is: temperature 25 ℃, calcium hydroxide contains solid The mass fraction is 10%, the CO2 volume fraction is 99.9%, and the stirring speed is 400r/min.
(2) Calcium carbonate is modified with sodium stearate, the modification effect is the best when the amount of modifier is 3%, the temperature is 80°C, the reaction time is 30min, and the stirring speed is 700r/min.
(3) Application tests show that modified calcium carbonate can effectively improve the mechanical properties of PVC products and reduce the cost of PVC application.
Hemostasis, antibacterial, drug carrier, kaolin has infinite potential in the field of biomedicine!

Mineral materials are widely used in biomedicine and have a long history.
1. Hemostatic material
"Compendium of Materia Medica" records: Baishizhi with kaolin as the main component can be used to absorb toxic substances, astringe and solidify, stop bleeding, and suppress secretion. In 2006, the American company Z-Medica developed a kaolin hemostatic product called "war-wound gauze", which is applied to special parts where tourniquets cannot be used. It is portable, easy to use and efficient, and has a shelf life of 5 years.
A new type of iron oxide/kaolin nanoclay composite was successfully synthesized based on the natural hemostatic agent in place of ochre to control hemorrhage. The morphology of the oxide has a significant effect on its hemostatic effect.
The in vitro hemostatic properties of Quikclot, a traditional commercial zeolite-based hemostatic agent, and layered silicates were compared, and the results showed that layered silicates (synthetic hydrotalcite, series of montmorillonite, kaolinite) clay minerals did not release during in vitro hemostasis. Heat, and extensive coagulation properties, both low price, stable and non-toxic properties, can be used as a new coagulant to replace QC.
A graphene-kaolin composite sponge gel (GKCS) was synthesized by hydrothermal method. The results of the rabbit artery injury experiment showed that the hemostasis time of the complex was 73±12s, and the hemostatic performance was significantly improved. In practical application, kaolinite-impregnated gauze was used for hemostasis after tonsillectomy, and 84.8% of patients had complete hemostasis after 5 minutes, while only 34.8% of patients with traditional standard postoperative gauze had hemostasis.
2. Drug carrier
Kaolin has a simple composition and is a typical natural 1:1 layered silicate mineral with a large diameter-to-thickness ratio, small size, and good biocompatibility. Therefore, kaolin can be used as a carrier to achieve drug loading and release. However, due to its weak ion exchange capacity, drug molecules can only be adsorbed on the surface of kaolin, and it is difficult to enter the interlayer, and the combination is not tight enough, and the drug loading effect is greatly affected.
Using the kaolin after methanol intercalation as the carrier, compared with the unmodified kaolin, after loading the small molecule chemotherapeutic drug 5-fluorouracil, it was found that the loading of the modified kaolin was as high as 55.4%, which was 147.3% higher than that of the unmodified kaolin. . This is because the grafting of methoxy groups between the kaolin layers expands the kaolin layer spacing, provides new active sites for drug molecules, and promotes the entry of drugs into the interlayer.
3. Antibacterial material
The epoxifloxacin was adsorbed on the surface of kaolinite, and the maximum adsorption amount was reached after 1 h. Compared with montmorillonite, kaolinite has a weaker ion exchange capacity, so the antibacterial agent is easier to release and has a better bactericidal effect. By measuring the adsorption capacity of CPB, it was found that CPB-kaolinite has antibacterial activity when [CPB] exceeds its CMC value. When the loading of CPB on kaolinite is high, the overall charge changes from positive to negative, so it has the ability to adsorb and kill bacteria. Therefore, kaolin can be well used for sterilization, and in the development of organoclay as an antibacterial agent, the amount of surfactant fixed on the clay must exceed the CMC value.
4. Tissue Engineering
Three-dimensional mesoporous bioglass (3D MBG) scaffolds with mesoporous structures and highly interconnected macroporous networks are considered ideal biomaterials for bone tissue applications. However, its inherent brittleness and poor mechanical strength seriously affect its performance and clinical application.
A three-dimensional MBG scaffold with excellent mechanical strength, mineralization ability and good cellular response was successfully prepared by using kaolin as a binder and a modified polyurethane foam (PU) template method. The developed hybrid MBG-xk has a porosity of 85%. With the increase of kaolin content (5%-20%), the compressive strength is between 2.6-6.0MPa, which is about 100 times that of the traditional PU-template MBG scaffold. After adding kaolin, the pH environment of MBG-10K scaffold was more stable and ideal, and the protein adsorption capacity was enhanced.
In the future, the research on the structure and performance mechanism of kaolin will be more in-depth and microscopic, and kaolin will play a greater role in more emerging fields.
What are the high-end application fields of porous calcium carbonate?

Porous materials are a class of materials with special properties, generally with large specific surface area, good thermal stability, chemical stability and biodegradability, and a suitable degradation rate, which makes the material suitable for use in many fields such as medicine, electronics, and ceramics. It can be widely used and is a very promising functional material.
1. Drug carrier
Drug carriers are an important part of targeted drug delivery, especially in the treatment of some major diseases (such as cancer, hyperglycemia, etc.). The substance selected as the drug carrier should not only be able to load a sufficient amount of drugs without reacting with it, but also be able to fully release the drug under specific conditions to exert its efficacy, and at the same time, the carrier itself should be non-toxic and stable in nature, etc. Require. Traditional carriers are often difficult to decompose, toxic or have small pore capacity.
Using porous calcium carbonate as a carrier can not only effectively solve the above problems, but also can be directly used as a drug to supplement calcium, inhibit gastric acid, and the like. Therefore, in recent years, there have been more and more studies on the application of porous calcium carbonate in drug delivery at home and abroad.
2. Bioceramics
Calcium carbonate is widely used in biology and medicine because of its good osteogenic and osteoinductive activity, biocompatibility and degradability. Using natural resources with high calcium carbonate content such as natural coral as raw materials, the new porous calcium carbonate ceramic PCCC prepared by various methods such as salting out method can be made into cell scaffolds. It has been used as human bone marrow cells, In vitro culture of fibroblasts, gingival fibroblasts and fetal rat osteocytes. Clinically, orthopedics and oral and maxillofacial surgery use PCCC for the repair of bone defects, and have achieved good results.
3. Waste paper recycling
While the whole country attaches great importance to the supply-side reform, environmental protection is also paying more and more attention. In the field of environmental protection, the degree of recycling of waste paper has reached an unprecedented level. Asia's waste paper consumption accounts for half of the global waste paper consumption, and its consumption in 2015 was about 103 million tons, far exceeding that of Europe and the United States. However, in terms of the key technology of recycling waste paper, due to the late start of China's development and insufficient investment in the early stage, the technology is relatively backward and the scope of utilization of recycled paper is narrow.
4. Superhydrophobic surface material
Super-hydrophobic material, also known as imitation lotus leaf surface material, is a special material with a stable surface contact angle greater than 150° and a rolling contact angle less than 10°. The preparation of superhydrophobic materials is mainly affected by their surface, so it is the key to develop superhydrophobic surface materials.
5. Biosensors
Biosensors are rapid and trace analysis methods at the molecular level of substances, and have broad application prospects in clinical diagnosis, industrial control, food and drug analysis, environmental protection, and biotechnology research.
6. Biological microcapsules
Biological microcapsules originated in the 1950s, mainly encapsulating biologically active substances in microcapsules with selectively permeable membranes, and is the main technical means for immobilizing biological substances (cells, enzymes, etc.). Among the preparation methods of microcapsules, the template method is the most commonly used, and the templates usually used are all porous materials. In recent years, due to the strong development momentum of porous calcium carbonate, scientific researchers have also applied it to the preparation of biological microcapsules.
7. Other
Porous calcium carbonate is not only used in the above-mentioned fields, but also has good performance in many other aspects.
Artificial quartz stone industry has broad prospects

Building decorative stone can be divided into two categories: natural stone and artificial stone. As a kind of resin-type artificial stone, artificial quartz stone is made of unsaturated polyester resin (UPR) as the binder and quartz sand and quartz powder as the main filling materials.
Artificial quartz stone inherits the characteristics of natural granite, which is hard, corrosion-resistant, wear-resistant and beautiful in appearance, and overcomes the shortcomings of natural stone, such as non-renewable, poor stain resistance, and radioactivity in some types, so it is widely used in With kitchen, sanitary and traditional architectural decorative stone has the advantages of zero formaldehyde, no radiation, moderate hardness, good stain resistance, clean and environmental protection.
Artificial quartz stone is a new type of building decoration material that appeared relatively late. In recent years, with the maturity of production and manufacturing technology and the significant improvement of design and color design capabilities, the market share of artificial quartz stone has increased significantly. According to Freedonia statistics, from 1999 to 2016, the global sales of artificial quartz stone to end consumers increased at a compound annual growth rate of 17.9%, which was significantly higher than the overall 4.9% compound annual growth rate of surface materials. The surface material forms a certain degree of substitution effect.
Global fluorite resources are unevenly distributed, and production has increased in the past five years

Fluorite, also known as fluorite, is mainly composed of calcium fluoride. The calcium atoms are coordinated with eight surrounding fluorine atoms, and the fluorine atoms are surrounded by four calcium atoms to form an ideal tetrahedron. The crystal structure of fluorite will directly affect its surface properties, affect the effect of chemicals and fluorite, and is related to the purification of difficult-to-handle fluorite. From the perspective of the structure of fluorite, there are "holes" in its crystal structure, which are easily filled by other ions, so it has various colors, such as green, yellow, purple, white, blue, black and other colors.
The total global fluorite reserves are 320 million tons, but the distribution is uneven, with Mexico, China, South Africa and Mongolia accounting for more than half of the fluorite reserves. First of all, in terms of total volume, global fluorite reserves will grow steadily from 2010 to 2022. According to the world fluorite reserves data released by the US Geological Survey in 2022, the world's total fluorite reserves will be 320 million tons by the end of 2021 (equivalent to fluoride Second, in terms of distribution, fluorite resources are mainly distributed in Mexico, China, South Africa, and Mongolia. By the end of 2021, its fluorite reserves will be 68 million tons, 42 million tons, 41 million tons, and 22 million tons respectively, accounting for The global fluorite reserve ratios are 21.25%, 13.13%, 12.81%, and 6.88%, respectively. However, the United States, the European Union, Japan, South Korea and India have almost few fluorite resources and reserves. Worldwide, the distribution of fluorite is structurally scarce.
In the past five years, global fluorite production has increased year by year. China, Mexico and Mongolia have the world's top three fluorite production, accounting for more than 80%. First, in terms of output, global fluorite production has grown steadily in the past five years. According to the world fluorite production data released by the US Geological Survey in 2022, the world’s total fluorite production will be 8.6 million tons by the end of 2021; Look, in 2021, China, Mexico, and Mongolia will be the world's largest fluorspar producers, with their fluorspar output of 5.4 million tons, 990,000 tons, and 800,000 tons respectively, accounting for 63%, 11%, and 9% of global fluorspar production, respectively. %, while Germany, Iran, Pakistan, the United States and other countries produce less fluorite. Worldwide, there is a structural imbalance in fluorite production.
Fluorite is widely used in information technology, new energy, high-end manufacturing and other fields, and has an irreplaceable strategic position. In the field of information technology, hydrogen fluoride and fluorine-containing special gases are cleaning agents and etching gases for integrated circuits, semiconductors, etc.; in the field of new energy, fluorite is used in the production of cathode materials and electrolytes for lithium batteries, and is also used for uranium enrichment and purification. Necessary raw materials; in the field of new materials, fluorite downstream product fluorine silica gel is used in the tight sealing of vehicles, and high-performance fluorine materials are used in key fields such as aerospace and photovoltaic power generation; in addition, fluorite is also used in biological fields, High-end manufacturing and energy conservation and environmental protection are the upstream raw materials for many high-tech industries and have an irreplaceable strategic position.
Effects of Aluminum Hydroxide Modification on Properties of Natural Rubber

Aluminum hydroxide flame retardant has played an important role in the field of polymer flame retardant due to its advantages of smoke suppression, flame retardant, non-toxic, non-volatile and low price, and its dosage is far ahead of other flame retardants.
Ultrafine aluminum hydroxide is a product with a regular crystal structure produced by a special production process. It has the advantages of high purity, small particle size, good crystal form, low surface activity, and small specific surface area. It can be filled in large quantities in rubber and plastics. Applicable to all kinds of processing technology.
Its flame retardant principle is that a large amount of crystal water is released during the thermal decomposition process. Because the evaporation of crystal water needs to absorb a lot of heat, it plays the role of cooling the polymer material; the generated water vapor can dilute the flammable gas and inhibit the spread of combustion; new The generated metal oxides have high activity and can adsorb solid particles and play a role in suppressing smoke. In addition, the metal oxides covering the surface of the polymer material can promote the formation of carbon on the surface of the substrate and prevent the spread of flame.
However, due to the extremely strong polarity and hydrophilicity of aluminum hydroxide inorganic flame retardants, it has poor compatibility with non-polar polymer materials. In order to improve the compatibility between aluminum hydroxide and polymers, it is usually necessary to For surface treatment, one of the most effective methods is to use a coupling agent for surface treatment of aluminum hydroxide.
Using natural rubber as the base material, the effects of superfine aluminum hydroxide surface treatment on the mechanical properties and flame retardant properties of vulcanized rubber before and after surface treatment were studied. The results show that:
(1) When the superfine aluminum hydroxide flame retardant natural rubber, the mechanical properties decrease obviously with the increase of the addition amount. When the addition amount reaches 150 parts, the flame retardant reaches the FV0 level, the oxygen index reaches 29%, and the smoke generation is small. Under the conditions of low smoke and low halogen, it can be properly considered to be synergistic with a small amount of halogen-based flame retardants to improve the mechanical properties.
(2) Surface modification treatment of ultrafine aluminum hydroxide with silane coupling agent can effectively improve the compatibility between aluminum hydroxide and natural rubber, improve the processing performance and the mechanical properties of vulcanizate, and the flame retardant performance changes relatively. Small. When the amount of silane coupling agent added was 1.5% of the mass of aluminum hydroxide, the performance improved the most.
(3) Under this formula system, within a certain range, the oxygen index of the vulcanizate increases by about 2 units for every 30 parts of superfine aluminum hydroxide added.
Development, technology status and future development trend of modified plastics industry

Due to the rapid development of the plastics industry, the filler masterbatch is no longer used as a single filler material. People use more advanced processes from the production methods of open refining and banburying, adding inorganic materials, chemical additives and other materials. Their respective characteristics and commonalities, and then the use of twin-screw extruders and triple-screw extruders for mixing and extrusion has become an important way and method for people to improve the special properties of plastic products. Plastic filling modification is the fastest growing in recent years. A new industry in the plastics industry.
1. Application of 8 major modified plastics downstream market
Automotive industry; Home appliance industry; Electronic and electrical industry; Machinery and equipment industry; Railway/military/medical/aerospace.
2. Five types of plastic modification methods
(1) Modified filling
The main purpose of filling masterbatch is to reduce production cost. Most of them are produced by using inorganic powder or industrial waste with low price and wide sources as filling material, and adding appropriate amount of additives and synthetic resin.
(2) Modified masterbatch
Modified masterbatch is a new modified material developed on the basis of filler masterbatch. Add inorganic materials such as glass fiber, talc, mica, wollastonite, barium sulfate, kaolin to the resin, or add synthetic resins or auxiliaries with special properties during processing, such as: anti-aging agent, antioxidant, anti-aging agent These composite materials play the functional characteristics of different materials in application.
(3) Functional modification
Various materials such as graphene, silicone powder, rare earth, magnesium hydroxide, metal fine powder (silver, copper, zinc, etc.) are added to the plastic, and the product index is improved through modification technology, and the flame retardancy, aging resistance, resistance Physical properties such as high and low temperature have been improved, and special properties such as electrical conductivity, antibacterial, insulation, and reinforcement can also be realized, and it has occupied a place in the main durable plastic product market.
(4) Multi-component compound modification
Multi-component composite modification mainly combines plastics with one or more inorganic materials, polymer materials, chemical additives, etc. through blending, grafting, block and other forms to make plastics "alloying". The properties of each component complement each other to form a plastic material with multiple excellent properties, so as to achieve the purpose of improving performance and multi-functionality.
(5) Special modification
Different functional materials or additives are added to the special plastics, so that the expensive special plastics not only maintain the original characteristics, but also have special functions, which are suitable for the market application of various products.
3. Three new trends in the development of modified plastics
(1) Nanoscale inorganic materials
Inorganic materials are widely used in plastics. The functions of inorganic materials are gradually highlighted with the ultra-fine particle size. Plastics modified with inorganic nano-powders have many unique properties, bringing new development opportunities to the development of the plastics industry.
(2) High-efficiency chemical additives
The development of new high-efficiency additives has become an important development direction for modified plastics. The additives involved in modified plastics are in addition to those commonly used in plastic processing, such as heat stabilizers, plasticizers, UV absorbers, nucleating agents, antistatic agents, In addition to dispersants and flame retardants, high-efficiency and multi-functional functional additives such as toughening, flame retardant, synergistic, and alloy compatibility (interface compatibility) are also critical to modified plastics.
(3) Environmental protection of modified plastics
With the enhancement of people's awareness of environmental protection and the increasingly strict environmental regulations, the concepts of environmental protection such as plastics' renewable utilization, environmental digestibility, biodegradability, non-toxicity, odorless, and pollution-free have been integrated into the design and manufacture of modified plastics In the process, attention should be paid to the conservation and rational utilization of energy resources, and the research and development of non-polluting, fully degradable, recyclable and environmentally friendly modified plastic products has become a new hotspot.
Preparation technology of clay mineral-organic polymer composite bactericidal material
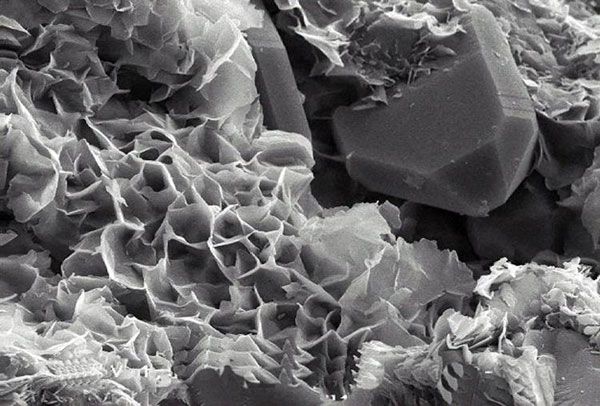
Among the new bactericidal materials based on clay minerals, clay minerals themselves are mainly used as carriers for loading bactericidal substances (such as metals, metal oxides, organic substances), and their bactericidal ability is still limited. A variety of methods are used to prepare modified clay minerals, and the composites made of clay minerals and other materials can be used as new bactericidal materials to produce bactericidal effects on a variety of bacteria.
Clay minerals can enhance the bactericidal ability through various modification methods (including thermal modification, acid modification, inorganic modification of metals or metal oxides, organic modification and composite modification, etc.). The surface area is increased, the mineral porosity and dispersibility are improved, and the overall thermal stability and mechanical strength of the material are improved. The clay minerals used for modification and preparation of bactericidal materials are mainly montmorillonite, kaolinite, halloysite, and vermiculite. is widely used for its adsorption capacity.
The preparation of clay minerals-organic polymer bactericidal materials usually refers to the addition of organically modified clay minerals to the organic polymer matrix to enhance the physicochemical properties and bactericidal activity of the materials. Such materials are mainly used to prepare nanofiber antibacterial cotton cloths, cotton pads and films. etc., clay minerals are used as fillers in composites to enhance the thermal and mechanical stability of nanomaterials, and clay minerals are usually at the nanoscale.
In view of the poor compatibility of clay minerals with organic molecules, organic compounds are often used to modify clay minerals to increase the dispersibility of clay minerals in organic solvents and ensure high compatibility between subsequent organic compounds and modified clay minerals. sex. Organic modification often uses anionic and cationic surfactants (quaternary ammonium salts and hybrid compounds are the most common), by changing the surface properties of the clay (changing the surface electrical properties and the surface hydrophobicity) or inserting organic matter into the interlayer (increasing the interlayer). domains and become hydrophobic between layers) to achieve modification. The organic polymer matrix used mainly includes polypropylene, polyethylene, polyethylene terephthalate, polyurethane, polystyrene, polyamide, polyolefin and the like. Biopolymers such as cellulose, starch, corn-derived plastics, polylactic acid, etc. have received attention due to their environmental friendliness and renewability.
Due to the high compatibility between organomodified clays and polymers, organomodified clays have become ideal materials for enhancing the properties of polymer matrices and are widely used as precursors for bactericidal materials. The properties of composite bactericidal materials are affected by the size scale of different components and the degree of mixing between multiple phases. During the preparation process, three types of intercalated composite materials, clay-exfoliated composite materials and flocculated composite materials are usually formed.
In intercalated nanocomposites, polymer chain moieties are regularly inserted between layers of clay. In exfoliated nanocomposites, the individual clay structural unit layers are relatively uniformly separated in the continuous polymer matrix, and the clay structural unit layers are completely exfoliated in the polymer matrix. The flocculated nanocomposite refers to the phenomenon of “flocculation” similar to the interaction of the hydroxylated edges between the clay mineral structural unit layers, the interlayer domain is reduced, and the polymer and the clay mineral phase are separated to a certain extent.
To study the bactericidal activity of Cu-loaded montmorillonite-chitosan nanocomposites. The synthesis of the composite is accomplished by ion exchange by placing montmorillonite in a medium containing copper sulfate. The lethality rate of the bactericidal material to Escherichia coli is as high as 99.98%, and all Staphylococcus aureus died after being treated with the material.
