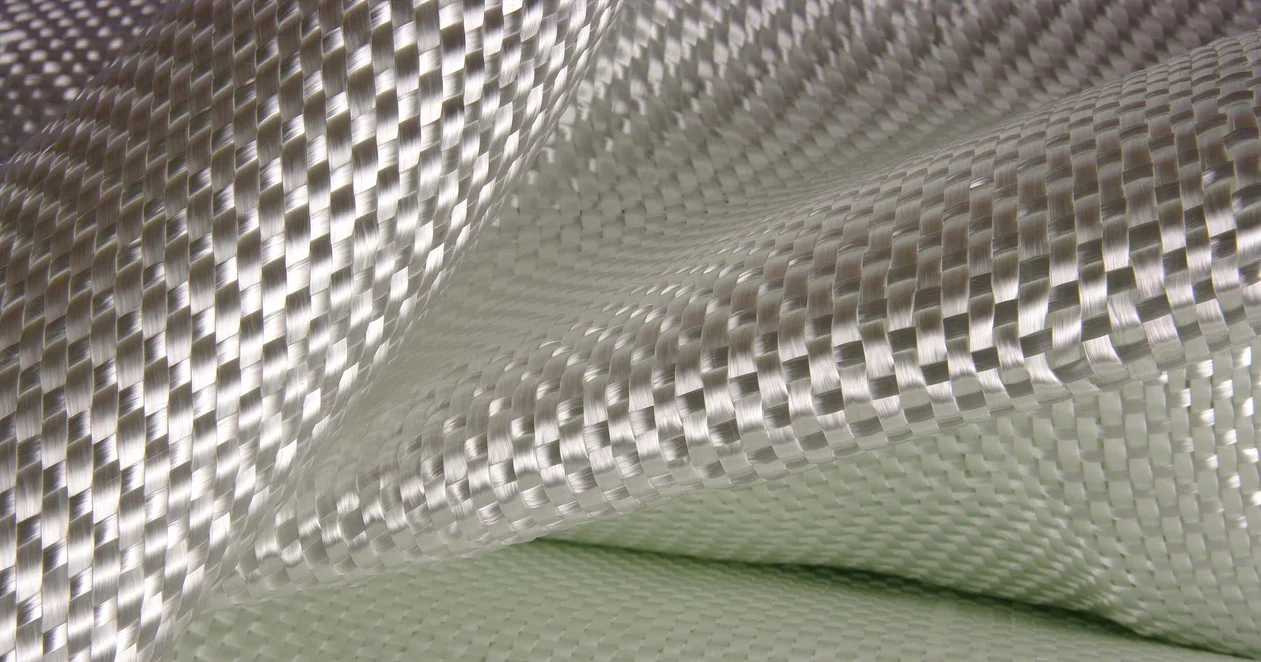
Glass fiber composite material properties
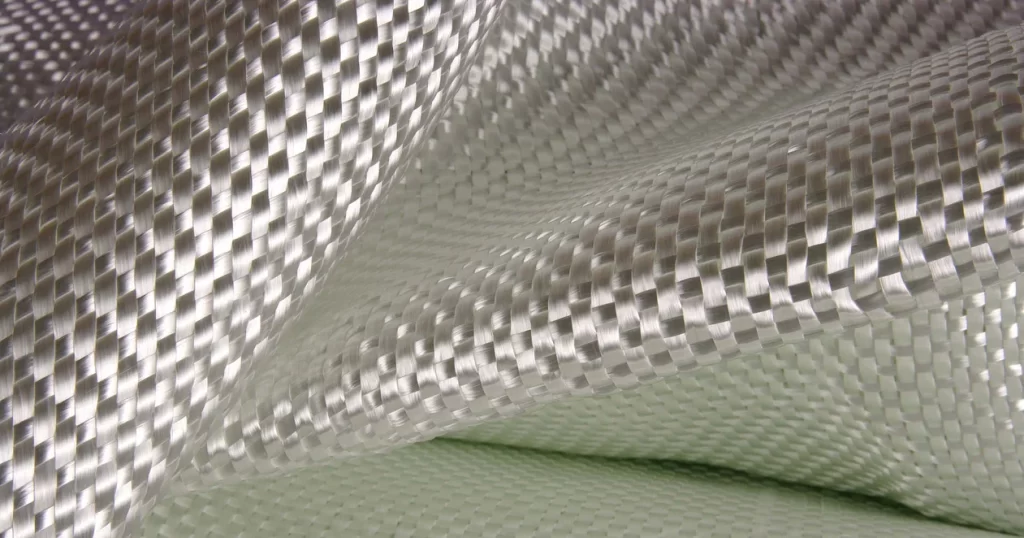
Fiberglass is a material composed of many extremely fine glass fibers. It is made by forcing molten glass through a sieve, which spins it into threads and then combines to form glass fibers.
Fiberglass composites are a reinforced plastic material consisting of glass fibers embedded in a resin matrix. Fiberglass composites have excellent specific strength, are light in weight but have mechanical properties close to metal; they are rust-proof and can withstand acid, alkali, moisture and salt spray environments for a long time, and have a longer service life than traditional metal materials; performance can be optimized by adjusting the fiber layup and resin type, and can be processed into complex shapes; they are non-conductive and transparent to electromagnetic waves, and are suitable for special functional components such as electrical equipment and radomes; compared with high-end composite materials such as carbon fiber, fiberglass is cheaper and is an economical high-performance material choice.
Glass fiber composite materials used in low altitude economy
Widely used in the field of drones
Fuselage and structural components: Glass fiber reinforced plastic (GFRP) is widely used in key structural components such as the fuselage, wings and tail of drones due to its lightweight and high strength.
Blade Materials: In drone propeller manufacturing, fiberglass is used in combination with materials such as nylon to increase rigidity and durability.
Important materials for electric vertical take-off and landing aircraft (eVTOL)
Fuse frame and wings: eVTOL aircraft have extremely high requirements for lightweight, and glass fiber reinforced composite materials are often used in combination with carbon fiber to optimize the fuselage structure and reduce costs.
Functional components: Glass fiber is also used in eVTOL avionics devices (such as RF power amplifiers), and its high temperature resistance and insulation properties make it an ideal choice.
As a strategic basic material in the low-altitude economy, glass fiber has broad application prospects in drones, eVTOL and other fields. With policy support and technological progress, its market demand will continue to grow and become an important force in promoting the development of the low-altitude economy.
The neglected gold: rare earth polishing powder

Rare earth cerium-based polishing powder is the mainstream rare earth polishing powder at present. It has excellent polishing performance and can improve the surface finish of products or parts. It is known as the "king of polishing powder". The glass processing industry and the electronics industry are the main downstream application fields of rare earth polishing powder. The waste of rare earth polishing powder that fails after polishing accounts for about 70% of the output each year. The waste components mainly come from rare earth polishing powder waste residue, waste liquid, glass fragments from polishing workpieces, grinding skin (organic polymer) from polishing cloth, oil and other impurities, and the proportion of rare earth components is 50%. How to dispose of the failed rare earth polishing powder has become a major problem for downstream application companies.
Currently, the commonly used methods for recycling rare earth polishing powder waste are physical separation and chemical separation.
Physical separation method
(1) Flotation method
In recent years, flotation technology has been widely used in solid waste treatment. Due to the difference in hydrophilicity of the components in waste rare earth polishing powder, different flotation agents are selected to improve the affinity of the components in aqueous solution, leaving the hydrophilic particles in the water, thereby achieving the purpose of separation. However, the size of the polishing powder particles affects the flotation recovery rate, and the recovery purity is not enough.
During flotation, different collectors are selected, and the impurity removal effect varies greatly. Yang Zhiren et al. found that when the pH of styrenephosphonic acid is 5, the recovery rate of cerium oxide and lanthanum oxide after flotation reaches 95%, while the recovery rate of calcium fluoride and fluoroapatite is only 20% at most. Particles with a diameter of less than 5 microns need to be further separated to remove impurities due to poor flotation effect.
(2) Magnetic separation method
Waste rare earth polishing powder has magnetism. Based on this, Mishima et al. designed a device with a vertical magnetic field to recover rare earth polishing slurry. When the flow rate of the waste powder slurry is 20 mm/s, the circulation time is 30 min, the slurry concentration is 5%, and the pH of the slurry is 3, the separation efficiency of cerium dioxide and iron flocculant can reach 80%. If the magnetic field direction is changed to a horizontal gradient and then MnCl2 solution is added, silicon dioxide and aluminum oxide with opposite magnetic properties can be separated from cerium dioxide.
(3) Other methods
Takahashi et al. froze the waste powder slurry whose particles were not easy to settle at -10°C, and then thawed it in an environment of 25°C. The impurities and rare earth oxides formed a layer, which facilitated the aggregation and recovery of useful substances in the waste.
Chemical separation method
The chemical method mainly adopts the recovery process after acid dissolution and alkali roasting, and uses a reducing agent as an auxiliary reagent to obtain rare earth polishing powder raw materials through impurity removal, extraction, and precipitation. This method has a high rare earth recovery rate, but the process is long and the cost is high. Excessive strong acid or strong alkali produces a large amount of wastewater. (1) Alkali treatment
Aluminum oxide and silicon dioxide are the main impurities in rare earth polishing powder waste. Use 4 mol/L NaOH solution to react with rare earth polishing powder waste for 1 hour at 60°C to remove silicon dioxide and aluminum oxide impurities in rare earth polishing powder waste.
(2) Acid treatment
When recovering rare earth elements from polishing powder waste, nitric acid, sulfuric acid and hydrochloric acid are often used for leaching. Cerium dioxide, the main component of rare earth polishing powder waste, is slightly soluble in sulfuric acid.
(3) Reducing agent assisted acid leaching
If CeO2 is directly leached with acid, the effect is not ideal. If a reducing agent is added to reduce Ce4+ to Ce3+, the rare earth leaching rate can be improved. Using the reducing agent H2O2 to assist hydrochloric acid leaching of rare earth polishing powder waste can significantly improve the experimental results.
Six process paths for high-purity quartz glass

Quartz glass has high purity, high spectral transmittance, low thermal expansion coefficient, and excellent resistance to thermal shock, corrosion, and deep ultraviolet radiation. It is widely used in high-end industrial manufacturing fields such as optics, aerospace, and semiconductors.
Quartz glass can be classified according to the preparation process. There are two main types of raw materials for preparing quartz glass. The first type is high-purity quartz sand, which is used for electric melting and gas refining to prepare fused quartz glass at high temperatures exceeding 1800°C; the second type is silicon-containing compounds, which are used to prepare synthetic quartz glass through chemical reactions.
Electric melting method
The electric melting method is to melt the powdered quartz raw material in the crucible by electric heating, and then form quartz glass through a vitrification process of rapid cooling. The main heating methods include resistance, arc and medium frequency induction.
Gas refining method
Industrially, gas refining method is slightly later than electric melting method. It uses hydrogen-oxygen flame to melt natural quartz, and then gradually accumulates it on the quartz glass target surface. The fused quartz glass produced by gas refining method is mainly used for electric light sources, semiconductor industry, spherical xenon lamps, etc. In the early days, large-caliber transparent quartz glass tubes and crucibles were directly melted with high-purity quartz sand on special equipment using hydrogen-oxygen flame. Now gas refining method is commonly used to prepare quartz ingots, and then the quartz ingots are cold or hot processed to make the required quartz glass products.
CVD method
The principle of CVD method is to heat volatile liquid SiCl4 to make it gaseous, and then let the gaseous SiCl4 enter the hydrogen-oxygen flame formed by the combustion of hydrogen and oxygen under the drive of carrier gas (O2), react with water vapor at high temperature to form amorphous particles, deposit on the rotating deposition substrate, and then melt at high temperature to form quartz glass.
PCVD method
The PCVD process was first proposed by Corning in the 1960s. It uses plasma to replace hydrogen-oxygen flame as the heat source for preparing quartz glass. The temperature of the plasma flame used in the PCVD process is much higher than that of ordinary flames. Its core temperature can be as high as 15000K, and the average temperature is 4000~5000K. The working gas can be appropriately selected according to the specific process requirements.
Two-step CVD method
The traditional CVD method is also called the one-step method or direct method. Since water vapor is involved in the reaction, the hydroxyl content in the quartz glass prepared by the one-step CVD method is generally high and difficult to control. In order to overcome this shortcoming, engineers improved the one-step CVD method and developed the two-step CVD method, also called the indirect synthesis method.
Thermal Modification
The thermal modification method first softens the quartz glass base material by heating it, and then obtains the desired product through methods such as trough sinking and drawing. In the thermal modification furnace, the furnace body is heated by electromagnetic induction heating. The alternating current passed through the induction coil in the furnace generates an alternating electromagnetic field in space, and the electromagnetic field acts on the heating element to generate current and heat. As the temperature rises, the quartz glass base material softens, and at this time, a quartz glass rod/tube can be formed by pulling down with a tractor. By adjusting the temperature in the furnace and the pulling speed, quartz glass rods/tubes of different diameters can be drawn. The coil arrangement and furnace structure of the electromagnetic induction heating furnace have a great influence on the temperature field in the furnace. In actual production, the temperature field in the furnace needs to be strictly controlled to ensure the quality of quartz glass products.
What are the types and wide applications of bentonite?

Bentonite is mainly divided into several types, such as sodium bentonite, calcium bentonite, hydrogen bentonite and organic bentonite, according to the difference of interlayer cations.
Sodium bentonite: It has excellent swelling, water absorption, adhesion and plasticity, and is the most widely used type of bentonite.
Calcium bentonite: Compared with sodium bentonite, its swelling and adhesion are slightly weaker, but the price is more economical, and it is suitable for some occasions with low performance requirements.
Hydrogen bentonite: It has special chemical properties and can exert unique properties under certain specific conditions, such as high temperature stability.
Organic bentonite: Through organic modification, it has better dispersibility, suspension and stability, and is suitable for high-end application fields.
Wide application of bentonite
The versatility of bentonite makes it play an important role in different fields, and its wide range of application fields is amazing.
Construction field: Bentonite is widely used in the production of building sound insulation and heat insulation materials, waterproof coatings, wall materials and other products due to its excellent expansion and adhesion, providing strong support for the green development of the construction industry.
Environmental protection field: Bentonite has a strong adsorption capacity and can adsorb harmful substances such as heavy metal ions and organic pollutants in water. It is an important material in the field of environmental protection. At the same time, bentonite can also be used in the construction of anti-seepage layers in landfills to effectively prevent the leakage of landfill leachate.
Metallurgical field: Bentonite is mainly used as a furnace lining material in the metallurgical industry. It is resistant to high temperature and erosion, and protects the furnace body from high-temperature slag.
Agricultural field: Bentonite has the function of improving soil structure and improving soil fertility. By adding bentonite, the air permeability and water retention of the soil can be improved, and crop growth can be promoted.
Foundry industry: Bentonite is used as a coating and adhesive in the foundry industry to improve the surface quality and strength of castings.
Food industry: Bentonite is mainly used for bleaching and purification in the food industry, such as decolorization of oils and fats, purification of sugar solutions, etc.
Oil drilling: Bentonite is an important raw material for oil drilling mud, which can adjust the viscosity, shear force and water loss of the mud and improve drilling efficiency.
Demand for bentonite powder making equipment
With the continuous expansion of bentonite application areas, the demand for bentonite powder making equipment is also increasing. When choosing grinding equipment, it is necessary to consider multiple factors such as equipment performance, production capacity, energy consumption and after-sales service.
When choosing mineral powder for plastics, look at these 11 indicators

Common mineral powder materials used in the plastics industry include calcium carbonate (heavy calcium, light calcium, nano calcium), talc, kaolin, wollastonite, brucite powder, mica powder, barite powder, barium sulfate and many other varieties. For the main purpose of filling increment, it can generally be used to tens to hundreds of phr. For the purpose of improving performance and reducing costs, it can generally be used to dozens of parts.
The properties of inorganic mineral fillers have many effects on plastic products, including physical and chemical composition and properties, particle size and distribution, particle shape and surface properties, as well as density, hardness, whiteness, etc., which have an impact on the performance and process parameter requirements of plastics.
1. Geometric shape characteristics
The influence of filler particles of different geometric shapes on the strength of their plastic products is generally fibrous> flake> columnar> cubic> spherical. Flake fillers help to improve the mechanical strength of products, but are not conducive to molding processing.
2. Particle size and surface characteristics
Generally speaking, the smaller the particle size of inorganic non-metallic mineral fillers, the better the mechanical properties of plastics when they are evenly dispersed. However, while reducing the particle size of filler particles, the processing technology becomes more complicated and the cost increases accordingly.
3. Specific surface area
The larger the specific surface area, the better the affinity between filler and resin, but the more difficult it is to activate the surface of the filler and the higher the cost. However, for filler particles of the same volume, the rougher the surface, the larger the specific surface area.
4. Density
Particles of different shapes have different particle sizes and distributions. When the mass is the same, the apparent density of particles with the same true density may not be the same due to different stacking volumes.
5. Hardness
High hardness can improve the wear resistance of products, but it will wear processing equipment. People do not want the benefits of using fillers to be offset by the wear of processing equipment. For fillers of a certain hardness, the wear intensity of the metal surface of the processing equipment increases with the increase of the filler particle size, and its wear intensity tends to be stable after a certain particle size.
6. Color
In order to avoid obvious changes in the color of the filled material matrix or adverse effects on the coloring of the matrix, most production requirements require the whiteness to be as high as possible.
7. Oil absorption value
The oil absorption value of the filler affects the amount of plasticizer used in the filling system and the processability of the material. Fillers with low oil absorption values have good processability of the filling system and are easy to mix with resins, which can reduce the amount of plasticizer used.
8. Optical properties
Some products can use the light absorption of fillers to increase the temperature, such as agricultural plastic greenhouses.
9. Electrical properties
Except for graphite, most inorganic mineral fillers are electrical insulators.
10. Chemical composition
The chemical activity, surface properties (effects), thermal properties, optical properties, electrical properties, magnetic properties, etc. of inorganic mineral fillers depend to a large extent on the chemical composition.
11. Thermochemical effect
Polymers are easy to burn, but most inorganic mineral fillers, due to their own non-combustibility, reduce the combustible substances after being added to the polymer matrix and delay the combustion of the matrix. Environmentally friendly flame retardant filler.
In short, the role of inorganic non-metallic mineral fillers in polymer composites can be summarized as increasing, enhancing and giving new functions. However, because inorganic non-metallic mineral fillers and organic polymers have poor compatibility, inorganic non-metallic mineral fillers are modified to improve their compatibility with organic polymers and avoid uneven stress dispersion caused by direct addition.
Characteristics and applications of three common silicon micropowder products

Silica powder is made from crystalline quartz, fused quartz and other raw materials through grinding, precision grading, impurity removal and other processes to produce silicon dioxide powder.
1. Classification of silicon micropowder
According to the particle morphology, it can be divided into angular silicon micropowder and spherical silicon micropowder. According to the different raw materials, it can be divided into angular crystalline silicon micropowder and angular molten silicon micropowder. The performance and price of crystalline, molten and spherical silicon micropowders increase in turn.
Crystalline silicon micropowder is made of natural quartz blocks, quartz sand, etc. as raw materials, and is processed through grinding, precision grading, impurity removal and other processes.
Fused silicon micropowder is made of fused quartz, glass and other materials as the main raw materials, and is produced through grinding, precision grading and impurity removal.
Spherical silicon micropowder is made of selected angular silicon micropowder (made of quartz blocks/quartz sand, fused quartz blocks/quartz sand, glass materials) as raw materials, and is processed into spherical silicon dioxide powder materials by flame method. In addition, it can also be prepared by combustion and explosion method and liquid phase method.
2. Application of silicon micropowder
(1) Copper clad laminate
Ordinary copper clad laminates generally use angular silicon micropowder, which mainly plays a role in reducing costs. Some molten powders have better performance. For example, copper clad laminates with higher technical levels such as high frequency and high speed, HDI substrates, etc. generally use modified high-performance spherical silicon micropowder (usually with a median particle size of less than 3um).
For example, crystalline silicon micropowder has a simple process and low cost, and is mainly used for household copper clad laminates with relatively low requirements for product accuracy and density, signal transmission speed, etc.
Melted silicon micropowder has good performance, moderate cost, low dielectric loss and linear expansion coefficient, and can be used in copper clad laminates used in smart phones, tablets, automobiles, network communications and industrial equipment.
Spherical silicon micropowder has excellent properties such as good fluidity, low stress, small specific surface area and high packing density. High-frequency and high-speed copper-clad laminates such as supercomputers and 5G communications require low transmission loss, low transmission delay, high heat resistance and high reliability. Spherical silicon micropowder is needed as a key functional filler, and the powder impurity content is required to be low and the filling rate is required to be high.
(2) Epoxy molding compound
Generally, low-end and mid-end epoxy molding compounds mostly use angular silicon micropowder, while high-end epoxy molding compounds are mainly spherical silicon micropowder. Spherical silicon micropowder is beneficial to improve fluidity and increase filler dosage, reduce thermal expansion coefficient, and reduce wear of equipment and molds.
Zirconium silicate: the invisible giant in the high-tech era

With the rapid development of science and technology, new breakthroughs are constantly being made in the field of new materials. Among them, zirconium silicate, as an important inorganic material, not only plays a core role in the traditional ceramic industry, but also shows a wide range of application prospects in the high-tech field.
Zirconium silicate (ZrSiO₄) is a grayish white, water-insoluble inorganic substance with a theoretical composition of 67.1% ZrO₂ and 32.9% SiO₂.
It has a high melting point (2500 degrees Celsius), a high refractive index (1.93-2.01) and excellent chemical stability. These characteristics and the advantages they bring make zirconium silicate shine in many fields.
Significant whitening effect:
The baddeleyite formed by zirconium silicate in ceramic glaze can effectively scatter incident light waves, significantly improving the whiteness and glossiness of the glaze, and is an ideal material for ceramic whitening.
Strong chemical stability:
Zirconium silicate has extremely strong chemical stability and can resist the erosion of a variety of acids, alkalis and corrosive substances, ensuring that it can maintain stable performance in various harsh environments.
Excellent high temperature resistance:
The high melting point enables zirconium silicate to maintain its structure and performance stability in high temperature environments, making it an ideal raw material for preparing high-temperature ceramics and refractory materials.
Enhance glaze hardness and wear resistance:
The addition of zirconium silicate can significantly improve the hardness and wear resistance of ceramic glazes and extend the service life of products.
Environmentally friendly and pollution-free:
As an inorganic material, zirconium silicate is non-toxic and harmless, will not pollute the environment, and meets the requirements of modern green production.
Zirconium silicate is widely used in the production of architectural ceramics, sanitary ceramics, daily-use ceramics and handicraft ceramics due to its excellent opacity and the above advantages.
It can not only improve the bonding performance of ceramic body and glaze, but also improve the overall quality of glaze, making ceramic products more beautiful and durable.
Color picture tubes in the television industry:
The application of zirconium silicate in color picture tubes improves the clarity and color saturation of the displayed image, bringing a more realistic visual experience to the audience.
Emulsified glass:
In the glass industry, zirconium silicate is used as an emulsifier to help manufacture high-transparency and high-strength glass products, which are widely used in automobiles, construction and other fields.
High-performance materials:
Nano-scale zirconium silicate is an ideal choice for preparing high-end ceramics and functional materials, such as wear-resistant coatings and thermal insulation materials, due to its unique nano effect and the above advantages.
With the continuous advancement of science and technology and the enhancement of environmental awareness, the application field of zirconium silicate will be further expanded. In the future, we will see more high-performance and environmentally friendly zirconium silicate products come out, contributing more to scientific and technological progress and social development. In short, as an important inorganic material, zirconium silicate has shown great development potential in the ceramic industry and high-tech fields with its unique advantages and broad application prospects. We have reason to believe that in the future development, zirconium silicate will continue to play its unique advantages and become an important force to promote the progress of the industry.
Titanium Dioxide - One of the World's Best White Pigments

Titanium dioxide, also known as titanium dioxide, has the chemical formula TiO2 and is a white pigment with excellent performance. Nano titanium dioxide is an important type of inorganic functional material, also known as nano titanium dioxide. Nano titanium dioxide is a fine titanium dioxide powder made by a special process.
Application fields
1. Application in pigments and coatings
Pigment-grade titanium dioxide has high refractive index, strong tinting power, large hiding power, good dispersibility and whiteness, is non-toxic and has stable physical and chemical properties, and has excellent optical and electrical properties. It is widely used in latex paint, coil and iron printing coatings, automotive paints, powder coatings and other fields, accounting for more than 90% of all white pigments used, which can improve product quality, add color and brighten. Titanium dioxide with a particle size of 200~400nm also has functions such as ultraviolet shielding, electrostatic shielding, wear resistance and scratch resistance, improves coating adhesion and prevents sagging.
2. Application in textiles and chemical fibers
Textiles and chemical fibers are an important application field of titanium dioxide. It has a high refractive index, which makes it perform well in optical properties. Therefore, it is often used as a matting agent for synthetic fibers. Generally speaking, only 0.2%~0.5% of TiO2 needs to be added to synthetic fibers to obtain a significant matting effect.
3. Application in the papermaking industry
The papermaking industry is an important application field of titanium dioxide, which is often used for decorative paper, Bible paper and banknotes. Paper using titanium dioxide has the characteristics of high whiteness, high strength, good gloss, thin and smooth, and opaque printing. The opacity is much higher than that of calcium carbonate and talcum powder, and the weight is also lighter.
4. Application in cosmetics
TiO2 can absorb, reflect and scatter ultraviolet rays, and can play a role in protecting against ultraviolet radiation. It has certain application potential in the field of cosmetics. However, nano-TiO2 itself has a large specific surface energy, strong polarity, and is easy to agglomerate, which affects the actual application effect. Therefore, nano-TiO2 is usually surface-modified before being used in the cosmetics field.
5. Application in the plastics industry
The plastics industry is an important application field for titanium dioxide, and its consumption accounts for about 20% of the total. There are more than 50 special plastic titanium dioxide brands in the world. In addition to its high hiding power and color-reducing power, titanium dioxide can also improve the heat resistance, light resistance, and weather resistance of plastic products, and improve their mechanical and electrical properties.
6. Application in the ink industry
Titanium dioxide has good whiteness, small and uniform particle size, high refractive index, high tinting power and hiding power, good physical and chemical stability, light diffusion, light resistance, heat resistance, weather resistance and hydrophobicity, making it not only an indispensable white pigment in ink manufacturing, but also a necessary raw material for the preparation of many intermediate color ink products.
7. Application in the rubber industry
Titanium dioxide is used as a colorant in the rubber industry, and it also has the functions of filling, anti-aging, acid and alkali resistance and reinforcement. Adding titanium dioxide to white and light-colored rubber products will make the finished products have the characteristics of slow aging, high strength, no cracking, no fading, large elongation and acid and alkali resistance.
8. Application in medical and health care
TiO2 photocatalytic materials can destroy the cell walls and cell membranes of bacteria, thereby playing a role in sterilization and disinfection. Nano-TiO2 can decompose pathogens and endotoxins. TiO2 photocatalytic antibacterial building materials are used in places where bacteria multiply in large numbers, such as hospital wards and operating rooms, to degrade endotoxins on solid surfaces and in liquids at room temperature.
9. Application in batteries
Solar cells are a sustainable green energy source. Dye-sensitized solar cells (DSSCs) have low costs, relatively simple manufacturing methods, are non-toxic, harmless, and pollution-free, and have good development prospects. TiO2 can be used in the production of dye-sensitized solar cells. Adding nano-Au, Ag or Pt and other precious metal particles to the surface of TiO2 electrodes, doping with non-metallic ions and transition metal complexes can improve the photoelectric conversion efficiency of TiO2. TiO2 can also be used as an electronic buffer layer material in perovskite solar cells, as well as a negative electrode material for lithium-ion batteries and sodium-ion batteries.
Application of Ultrafine Powder Technology in Traditional Chinese Medicine Preparations

Ultrafine powder technology is a new chemical engineering technology that is currently popular in various countries. It began in the 1970s and has broad development prospects in the pharmaceutical industry. This article introduces the application of ultrafine powder technology in traditional Chinese medicine preparations and analyzes its impact on the quality and process of drug preparations.
At present, powders with a particle size of less than 3μm are called ultrafine powders abroad. Ultrafine powder technology refers to the preparation and use of ultrafine powders and related technologies. The research content includes the preparation, classification, separation, drying, surface modification, particle composite, particle size measurement, safety technology in the manufacturing and storage and transportation process of ultrafine powders. Ultrafine powder technology is also called ultrafine grinding technology and cell-level micro-grinding technology. This is a purely physical process. It can increase the median particle size of animal and plant medicinal powders obtained by traditional grinding technology from about 75 μm to below 5-10 μm. This technology has gradually been widely used in traditional Chinese medicine preparations, especially the use of ultrafine particles of drugs in external medicines, oral medicines and suspension injections. Therefore, the introduction of ultrafine powder technology in the pharmaceutical industry is inevitable for the development of traditional Chinese medicine. However, the use of ultrafine powder technology to micronize drugs will also have a certain impact on the quality of the drugs and the process of drug preparations.
In actual industrial production, the medicinal materials are often pre-treated by coarse grinding using traditional methods, and then further ultra-finely ground after screening to achieve the required particle size specifications (grading). The application of ultra-fine powder technology of traditional Chinese medicine has brought about the innovation and development of traditional Chinese medicine dosage forms, and broadened the dosage forms of crude drugs.
The key to the ultra-fine grinding process is to judge the appropriate grinding force field according to the physical properties of the crude drug, so as to select effective ultra-fine grinding equipment. At present, the ultra-fine grinding methods of traditional Chinese medicine mainly include mechanical grinding, vibration grinding and air flow grinding. There are many domestic ultra-fine grinding production industrial equipment for sale, including vibration mill, mechanical shearing mill, low-temperature mill, air flow mill. The latter two are widely used in the pharmaceutical industry, and among the air flow mills, the fluidized bed air flow ultra-fine mill is the most widely used.
Mechanical ultrafine grinding can be divided into dry grinding and wet grinding. According to the different principles of generating grinding force during the grinding process, dry grinding includes air flow type, high-frequency vibration type, rotating ball (rod) mill type, hammer type and self-grinding type. Wet grinding mainly includes colloid mill and homogenizer.
Modern ultrafine powder technology is a microscopic combination of drugs, making full use of micronization, compounding, precision, surface modification and particle design technology to make drugs reach a higher level. In this regard, there is a wide range of technical space for research and utilization. In-depth research and application of this technology will be a new technical growth point and a new economic growth point for traditional Chinese medicine.
6 common ultrafine grinding process flows, which one is suitable for your powder?
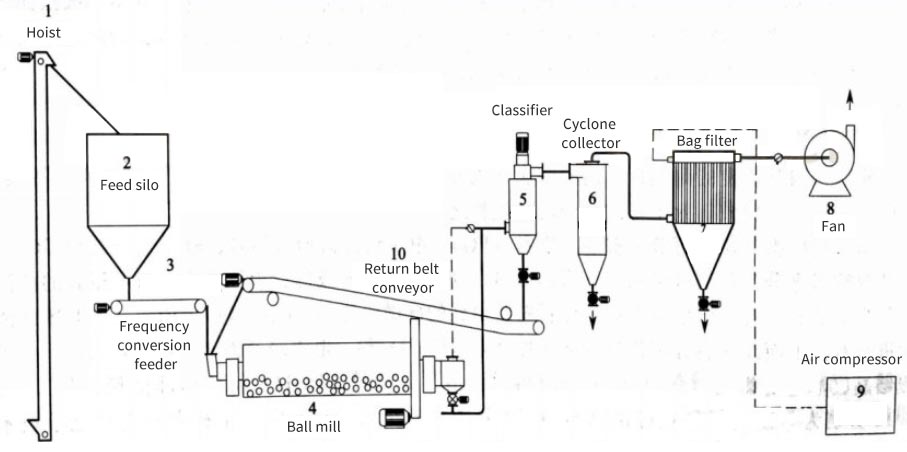
Impact ultrafine grinding process generally refers to the grinding and grading process for preparing powders with a particle size distribution of d97≤10μm, which is divided into dry method and wet method. At present, the ultrafine grinding unit operation (i.e. one-stage ultrafine grinding) used in industry has the following process flows:
1. Open circuit process
Generally, flat or disc-type, circulating tube-type and other air flow mills often use this open circuit process flow because they have the function of self-grading. In addition, intermittent ultrafine grinding also often uses this process flow.
The advantage of this process flow is that the process is simple, but for ultrafine grinders that do not have the function of self-grading, since there is no classifier in this process flow, qualified ultrafine powder products cannot be separated in time. Therefore, the particle size distribution range of general products is relatively wide.
2. Closed circuit process
Its characteristic is that the classifier and ultrafine grinder form an ultrafine grinding-fine grading closed circuit system. This process flow is often used for continuous grinding operations of general ball mills, stirred mills, high-speed mechanical impact mills, vibration mills, etc.
Its advantage is that it can timely separate qualified ultrafine powder products, thus reducing the agglomeration of fine particles and improving the efficiency of ultrafine grinding.
3. Open-circuit process with pre-grading
Its characteristic is that the material is first graded before entering the ultrafine grinder, and the fine-grained material is directly used as the ultrafine powder product. The coarse-grained material enters the ultrafine grinder for grinding. When the feed contains a large number of qualified ultrafine powders, this process can reduce the load of the grinder, reduce the energy consumption of the unit ultrafine powder product, and improve the operation efficiency.
4. Closed-circuit process with pre-grading
This combination of operations not only helps to improve the grinding efficiency and reduce the energy consumption per unit product, but also controls the particle size distribution of the product.
This process can also be simplified to only set up one classifier, that is, the same classifier is used for pre-grading and inspection and grading.
5. Open-circuit process with final classification
The characteristic of this grinding process is that one or more classifiers can be set after the grinder to obtain more than two products with different fineness and particle size distribution.
6. Open-circuit process with pre-classification and final classification
This process can not only pre-separate some qualified fine-grained products to reduce the load of the crusher, but also the final classification equipment can obtain more than two products with different fineness and particle size distribution.
How to set the number of ultra-fine grinding stages?
In terms of grinding methods, ultra-fine grinding processes can be divided into three types: dry (one or more stages) grinding, wet (one or more stages) grinding, and dry-wet combined multi-stage grinding.
The number of grinding stages mainly depends on the particle size of the raw materials and the required product fineness.
For raw materials with relatively coarse particle size, a process flow of first fine grinding or fine grinding and then ultra-fine grinding can be adopted. Generally, the raw materials can be crushed to 74μm or 43μm and then a stage of ultra-fine grinding process can be adopted;
For materials with very fine product particle size requirements and easy to agglomerate, a multi-stage ultra-fine grinding process flow can be adopted in series to improve operating efficiency.
However, generally speaking, the more grinding stages there are, the more complex the process flow and the greater the engineering investment.