Next-generation communication core material: lithium tantalate
With the rapid development of the Internet of Things, artificial intelligence, and big data technology, lithium tantalate (LiTaO3) has been widely used in digital signal processing, 5G communications, guidance, infrared detectors and other fields due to its excellent properties such as piezoelectricity, acousto-optics, and electro-optics. Its single crystal film is considered to be a new material urgently needed for the development of new devices in the post-Moore era.
Lithium tantalate is a multifunctional crystal material with excellent performance. It has an ilmenite structure and is colorless or light yellow. Its crystal raw materials are abundant, its performance is stable, and it is easy to process. It can produce high-quality, large-size single crystals. Polished lithium tantalate crystals can be widely used in the manufacture of electronic communication devices such as resonators, surface filters, and transducers. It is an indispensable functional material in many high-end communication fields such as mobile phones, satellite communications, and aerospace.
Main Applications
Surface Acoustic Wave (SAW) Filter
Surface acoustic wave filter is a special filtering device made by using the piezoelectric effect of piezoelectric crystal oscillator materials and the physical characteristics of surface acoustic wave propagation. It has the advantages of low transmission loss, high reliability, large manufacturing flexibility, analog/digital compatibility, and excellent frequency selection characteristics. Its main components include transmission line, piezoelectric crystal and attenuator. When the signal reaches the surface of the piezoelectric crystal through the transmission line, surface acoustic waves will be generated. The speed of surface acoustic waves of different frequencies is different during propagation. By reasonably designing the geometric shape and transmission parameters of the piezoelectric crystal and the interdigital transducer and the existence of the reflector, filtering effects of different frequencies can be achieved.
Crystal Oscillator
A crystal oscillator is an energy conversion device that converts direct current into alternating current with a certain frequency. It mainly uses the piezoelectric effect of piezoelectric crystals to generate stable electrical oscillations. When voltage is applied to the two poles of the chip, the crystal will deform, thereby generating voltage on the metal sheet. Crystal oscillators are widely used in communication radio stations, GPS, satellite communications, remote control mobile devices, mobile phone transmitters, and high-end frequency counters because of their highly stable frequency AC signals. It usually uses crystals that can convert electrical energy and mechanical energy to provide stable and accurate single-frequency oscillations. Currently, commonly used crystal materials include quartz semiconductor materials and lithium tantalate chips.
Pyroelectric detector
A pyroelectric detector is a sensor that uses the pyroelectric effect to detect temperature changes or infrared radiation. It can detect the energy changes of the target in a non-contact form, thereby generating a measurable electrical signal. Its core component is a pyroelectric chip, a single crystal material with special properties, usually composed of units with opposite charges, with crystal axes and spontaneous polarization. Pyroelectric materials need to be prepared very thin, and electrodes are plated on the surface perpendicular to the crystal axis. The upper surface electrode needs to be plated with an absorption layer before it can be used. When infrared radiation reaches the absorption layer, the pyroelectric chip will be heated and a surface electrode will be generated; if the radiation is interrupted, a reverse polarization charge will be generated.
Lithium tantalate has broad application prospects in 5G communications, photonic chips, quantum information and other fields due to its large pyroelectric coefficient, high Curie temperature, small dielectric loss factor, low thermal melting point per unit volume, small relative dielectric constant and stable performance.
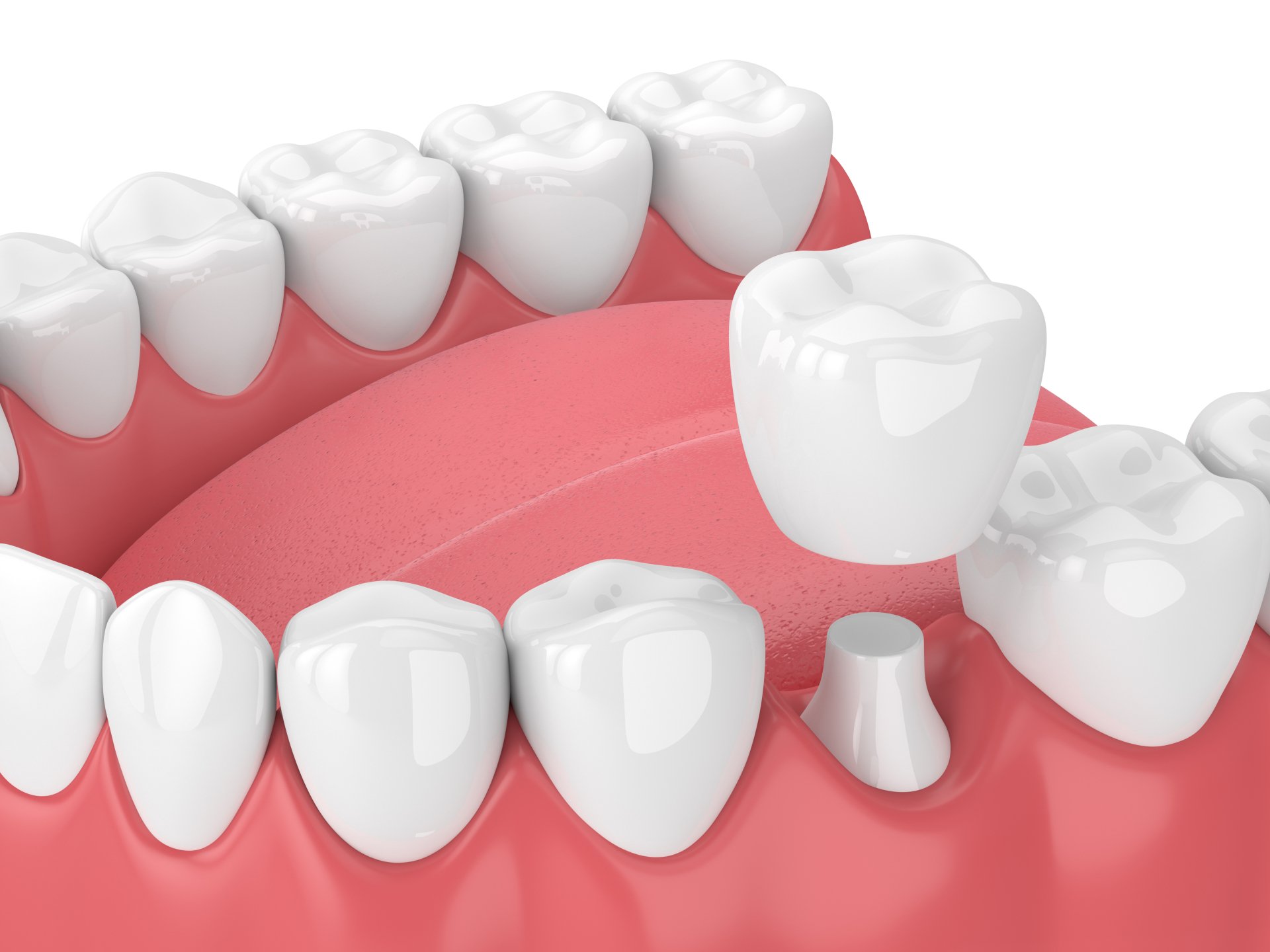
Ceramic materials used in tooth restorations
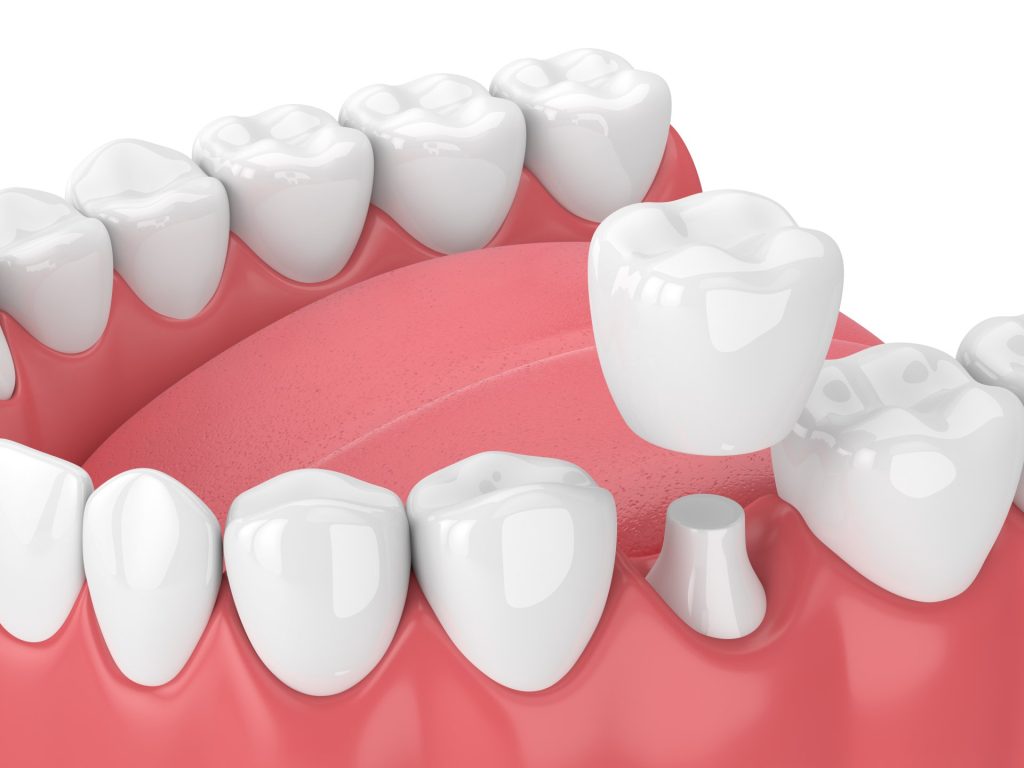
Dental restoration materials must undergo rigorous biological testing to ensure that they not only have the mechanical, physical, and chemical properties required for clinical use, but also have good biocompatibility. In recent years, with the continuous development of materials science and technology and the continuous improvement of people's living standards, ceramic materials, resin-based composite materials, metal materials, 3M nano-resins, glass-ceramics and other materials have gradually been widely used.
(1) Alumina ceramics
Alumina ceramics are white crystalline solids or powders with remarkable chemical stability and mechanical properties. As a dental restoration material, alumina has the color and light transmittance that match that of real teeth, meets aesthetic requirements, and has the advantages of weak toxicity to fibrous tissue in vitro.
(2) Zirconia ceramics
At the end of the 20th century, zirconia was developed as a dental restoration material. Zirconia ceramics have significant wear resistance, corrosion resistance, and high temperature resistance, good optical effects, are suitable for tooth restoration, and have high strength. Zirconia has strong stability and good biocompatibility. Compared with alumina, it has higher wear resistance and toughness. It is suitable for the production of valves, composite ceramic artificial bones, hip joints, bones, and tooth roots.
(3) Bioactive glass
Bioactive glass is an artificial biomaterial that can bond with bone tissue and connect with soft tissue at the same time. It has excellent properties such as biocompatibility, low toxicity, bone guidance and bone formation, and has good hemostasis and antibacterial effects. It can achieve specific biological and physiological functions when implanted in the body. Bioactive glass can be used as bone transplantation, bone filling material, alveolar ridge maintenance and reconstruction material, and oral implant coating material.
(4) Hydroxyapatite ceramics
Hydroxyapatite belongs to the hexagonal crystal system and is a typical bioactive ceramic. Its composition is close to the inorganic components of natural bone tissue and has good biocompatibility. It is not only safe and non-toxic when implanted in the body, but also can conduct bone growth. It is an excellent bioactive material. It is often used in the field of oral medicine for periodontal bone defect repair and artificial tooth root implants.
(5) Tricalcium phosphate ceramics
Tricalcium phosphate is an important calcium phosphate ceramic with good biocompatibility and biotoxicity. Tricalcium phosphate can be made into hollow structural components of a certain size and shape according to the requirements of degradation rate of different parts and different bone properties, and can be used to treat various orthopedic diseases. In addition, tricalcium phosphate has the biological characteristics of inducing periapical bone regeneration and pulp calcium bridge formation, and is widely used and valued in the field of oral medicine.
(6) Feldspar porcelain
Feldspar porcelain is a borosilicate feldspar glass with irregular grain structure distributed in the glass matrix. It is used in anterior tooth veneers, full crowns and posterior tooth inlays. It has good aesthetic effects and abrasion close to natural teeth. After grinding and polishing, it can be used in the mouth.
(7) Glass ceramics
Glass ceramics are polycrystalline solids with uniform and dense distribution of glass phase and crystal phase in a glass matrix obtained through a series of heat treatment procedures. They are also called microcrystalline glass. Glass ceramics have become the preferred material for aesthetic restoration of anterior teeth because of their transmittance and saturation close to natural teeth. Glass ceramics not only have excellent corrosion resistance and wear resistance, but also their flexural strength and fracture toughness can be controlled by adjusting the heat treatment process of the crystallization process. Therefore, products suitable for different uses have been developed one after another.
(8) Composite ceramics
Composite ceramics are a new type of resin-ceramic composite material that combines the characteristics of traditional ceramics with new resin process materials. Its advantage is that it can be realized using CAD/CAM technology. In addition, since composite ceramics contain a large amount of resin components, once the restoration is damaged, it is easy to repair it with resin.
The key to improving ball mill efficiency

Factors affecting grinding efficiency
Grinding efficiency is an important indicator of ball mill performance, which is crucial to improving mineral processing efficiency and reducing energy consumption.
Material properties are basic factors, and hardness, toughness, density and fracture characteristics affect the difficulty of grinding.
Mill operating parameters have a significant impact on efficiency, such as speed, filling rate, media size and type. Optimizing the speed can maximize the impact and friction, and the appropriate filling rate ensures effective contact between the material and the media. The type and size of the grinding media are also important. Media of different materials and sizes will affect the grinding efficiency. Choosing the right media can improve the grinding effect.
The choice of grinding process also affects the efficiency. Wet grinding is suitable for fine particle requirements, and dry grinding is suitable for materials with low water content.
The design and maintenance of the mill are also critical. The structural design affects the grinding efficiency, and improper maintenance will reduce the efficiency.
Ball mill speed
According to the kinetic energy theorem, when the mass of an object is constant, the greater the speed of the object, the higher the energy it carries. Similarly, the greater the speed of the ball mill grinding jar, the greater the crushing and grinding energy carried by the particle media particles, and the better the crushing and grinding effect, but there may be problems such as increased energy consumption, increased loss of the particle media itself, and severe heating in the grinding jar; if the grinding jar speed is too low, the energy carried by the particle media may not be enough to achieve the crushing and grinding of the material, and it will not play a grinding role.
Filling rate of particle media
The filling rate refers to the ratio of the internal volume of the grinding jar occupied by the particle media in a loose state to the actual volume of the grinding jar. The filling rate of the particle media in the grinding jar is one of the key factors affecting the grinding efficiency.
Particle size of particle media
According to the impulse equation of the object, objects of different masses carry different kinetic energy at the same speed. In the particle media of the same material, the particle size determines the mass of a single particle. Therefore, choosing the appropriate particle size of the particle media can effectively improve the grinding efficiency.
Ball ratio
The ball ratio is the ratio of the material to the grinding medium, which also has a significant impact on the grinding efficiency. An appropriate ball ratio can ensure that the grinding medium effectively transfers energy to the material. The determination of the ball ratio needs to consider the material characteristics, mill type and expected grinding fineness.
Grinding water volume
During the wet grinding process, the grinding water volume has a direct impact on the grinding efficiency and slurry concentration. The fluidity of the slurry needs to be controlled by adjusting the water volume to ensure good interaction between the medium and the material, while avoiding overloading the mill and reducing the grinding efficiency.
Steel ball size and ratio
In the operation of the ball mill, the steel ball is the grinding medium, and its size and ratio have a decisive influence on the grinding efficiency. Appropriate steel ball size and ratio can effectively improve the grinding efficiency of the material, reduce energy consumption, and extend the service life of the mill.
Improvement of process and equipment
Another key means to improve the operation rate of ball mill is the improvement of process and equipment. With the continuous development of modern technology and the progress of materials science, traditional ball milling process and equipment are facing the necessity of upgrading and transformation.
Fault analysis and prevention
The operating efficiency and stability of the ball mill directly affect the quality and efficiency of the entire production process. However, in the long-term operation process, due to the influence of various internal and external factors, the ball mill often has various faults, such as high main bearing temperature, abnormal running sound, bulging belly and other problems, which will not only affect production efficiency, but also may cause equipment damage and increase production costs.
How does barium sulfate play an important role in battery production?

The main component of barite is barium sulfate (BaSO4), and its most well-known uses are oil drilling mud weighting agents, barium chemicals, and raw materials for nuclear radiation protection.
Barium sulfate has the advantages of strong chemical inertness, good stability, acid and alkali resistance, moderate hardness, high specific gravity, high whiteness, and the ability to absorb harmful rays. It is an environmentally friendly material. High-purity nano barium sulfate not only has the uses of ordinary barium sulfate, but also has other special uses. For example, it is widely used in industrial sectors such as coatings, papermaking, rubber, ink, and plastics.
Barium sulfate also has an important use - the most commonly used inorganic expander in battery manufacturing. As a basic, renewable and recyclable new energy, batteries are widely used in various fields such as transportation, communications, electricity, railways, national defense, computers and scientific research.
As a new energy mineral, barium sulfate plays a very important role in battery production. The main reason for the shortened battery life is: sulfation of the negative plate of the battery. Therefore, in lead-acid batteries, the main role of barium sulfate is to enhance the activity of the negative plate, prevent the plate from hardening, and extend the service life of the battery.
In the negative lead paste of the battery, precipitated barium sulfate with excellent filling properties and stable properties is generally used to reduce the degree of sulfation of the negative electrode of the battery. The reasons are as follows:
1. Barium sulfate and lead sulfate have the same lattice structure, which is conducive to the lead sulfate (PbSO_4) produced by the negative electrode of the battery with the help of barium sulfate (BaSO4) to be evenly distributed in various positions of the plate, thereby inhibiting irreversible sulfation and extending the life of the battery.
2. The precipitated barium sulfate has a small particle size and good dispersibility. Experiments have shown that in the absence of agglomeration, the smaller the particle size of barium sulfate, the lower the degree of sulfation of the negative electrode of the battery.
3. Precipitated barium sulfate is of high purity, contains almost no iron, and is not easy to discharge. When the battery is discharged, PbSO4 can have more crystal centers, better prevent the lead specific surface area from shrinking, enhance the activity of the negative electrode plate, prevent the plate from hardening, and extend the service life of the battery.
4. Barium sulfate is extremely inert and does not participate in the redox process of the electrode. It mechanically separates lead from lead or lead sulfate, thereby maintaining a well-developed specific surface area of the electrode material.
High thermal conductivity fiber: opening a new era of thermal management

In today's era of rapid technological development, thermal management issues have become one of the key challenges faced by many fields. From the heat dissipation needs of electronic equipment to the temperature regulation of functional clothing, from thermal protection in aerospace to thermal conduction optimization in the field of new energy, high thermal conductivity fibers have gradually become the focus of research and industry with their unique performance and broad application prospects.
With the rapid development of aerospace, electronic chips, artificial intelligence and other fields, the application needs of high-power heat dissipation and heat dissipation have put forward higher and higher requirements for high thermal conductivity materials. High thermal conductivity fibers, such as mesophase pitch-based carbon fibers, boron nitride fibers, carbon nanotube fibers, graphene fibers, etc., not only show excellent high thermal conductivity, but also have high mechanical strength, directional thermal conductivity, and weavability. They are ideal materials for structural and functional integration of high-power heat dissipation applications.
1. Excellent thermal conductivity: The most notable feature of high thermal conductivity fiber is its excellent thermal conductivity. Compared with traditional fibers, high thermal conductivity fibers can transfer heat more quickly, effectively reduce local temperature, and improve heat conduction efficiency. This feature gives high thermal conductivity fibers unique advantages in heat dissipation and heat conduction.
2. Good mechanical properties: In addition to thermal conductivity, high thermal conductivity fibers usually have good mechanical properties, such as high strength, high toughness, and wear resistance.
3. Lightweight and flexible: High thermal conductivity fibers usually have a lighter weight and good flexibility, and can be woven, woven or composited according to different needs to make materials of various shapes and structures.
4. Chemical stability: High thermal conductivity fibers generally have good chemical stability and can maintain their stable performance under different chemical environments. This allows high thermal conductivity fibers to be used in various harsh working conditions, such as high temperature, high pressure, corrosive environment, etc.
Application areas of high thermal conductivity fibers
1. Heat dissipation of electronic equipment: As the performance of electronic equipment continues to improve, its heat dissipation problem has become increasingly prominent. High thermal conductivity fibers can be used as heat dissipation materials and applied to radiators, heat sinks and other components of electronic equipment to effectively improve the heat dissipation efficiency of electronic equipment, reduce operating temperatures, and extend the service life of equipment.
2. Functional clothing: High thermal conductivity fibers can be used in functional clothing, such as sportswear, outdoor clothing, etc., to achieve the regulation of human body temperature. In a cold environment, high thermal conductivity fibers can quickly transfer the heat generated by the human body, keep the temperature inside the clothing in a relatively stable state, reduce the accumulation of heat inside the clothing, thereby avoiding sweating due to overheating, and then preventing sweat from making the human body feel cold in a low temperature environment; in a hot environment, high thermal conductivity fibers can quickly transfer external heat to the surface of the human body, dissipate heat through sweat evaporation, and keep the body cool.
3. Aerospace: In the field of aerospace, high thermal conductivity fibers can be used as thermal protection materials in the outer shell, engine and other parts of aircraft, effectively reducing the heat generated by aircraft during high-speed flight and improving the safety and reliability of aircraft. In addition, high thermal conductivity fibers can also be used in electronic equipment heat dissipation, satellite thermal control and other aspects in the field of aerospace.
4. New energy field: In the field of new energy, high thermal conductivity fibers can be used as battery separators, electrode materials, etc. to improve the charging and discharging efficiency and safety of batteries. In addition, high thermal conductivity fibers can also be used in the thermal management of new energy equipment such as solar cells and fuel cells to improve the performance and stability of equipment.
Application fields of conductive carbon black

Conductive carbon black is a typical special carbon black with a conductivity generally in the range of 10-1~10-2S/cm. Conductive carbon black has the advantages of high electrical conductivity and thermal conductivity, low production cost, oxidation stability and low density, and has obvious advantages over metal powder or fiber fillers.
Battery field
Lithium-ion battery:
LiFePO4, LiNiO2, LiCoO2 and other positive electrode active materials are semiconductors or insulators with conductivity of only 10-9~10-3S/cm. Conductive additives need to be added to enhance their conductivity.
During the charge and discharge process, the negative electrode material will repeatedly expand and shrink due to Li+ insertion/extraction, which destroys the insertion channel of Li+ and reduces the discharge capacity.
Other batteries:
Nickel-hydrogen battery: Applied to the negative electrode, as an electron carrier and conductive additive, it helps the negative electrode material to better carry out electrochemical reactions, reduce electrode polarization, improve the battery's charge and discharge performance and cycle life, and reduce side reactions such as gas generation.
Nickel-cadmium battery: Acts on the negative electrode to improve the conductivity of the negative electrode material, reduce the internal resistance of the battery, improve the large current discharge capacity and charge and discharge efficiency, and reduce energy loss and heat generation.
Rubber and plastic products field
Antistatic products:
Manufacturing antistatic rubber products, such as antistatic rubber sheets, antistatic conveyor belts, rubber soles, medical rubber products, etc., can effectively prevent the generation and accumulation of static electricity and avoid the harm of static electricity to equipment and personnel.
Production of conductive plastic products, such as conductive films, conductive fibers, conductive leather products, etc., has important applications in electronic packaging, electromagnetic shielding and other fields.
Ordinary rubber and plastic products: It can improve the conductivity of rubber and plastic, make them have certain antistatic properties, reduce the impact of static electricity on products, and improve the mechanical properties and processing properties of materials.
Cable material field
Power cable shielding material
Anti-electromagnetic interference: In power cables, conductive carbon black can be added to the shielding layer of the cable to effectively shield external electromagnetic interference and ensure that the power signal transmitted by the cable is stable and accurate.
Homogenized electric field distribution: During the operation of the cable, the uneven distribution of the internal electric field may cause problems such as partial discharge, affecting the service life and safety of the cable.
Semi-conductive cable materials
Semi-conductive shielding layer: Semi-conductive shielding layer used for medium and low voltage cables. Conductive carbon black can be mixed with base materials such as rubber or plastic to form a semi-conductive composite material.
Improving processing performance: The addition of conductive carbon black can improve the processing performance of cable materials, making them easier to extrude and shape, and improving production efficiency and product quality.
Electronic printing and coating industry
Conductive ink and conductive coating:
Adding conductive carbon black can make inks and coatings conductive, and they are used in printed circuit boards (PCBs), electronic displays, electromagnetic shielding coatings and other fields.
Electronic printing:
Conductive carbon black can be used to make anti-static clothing, smart textiles, etc. In some working environments that require anti-static, wearing anti-static clothing can avoid static electricity damage to electronic equipment and harm to the human body.
Other fields
Fuel cells: In polymer electrolyte fuel cells, conductive carbon black can be used in the fuel electrode and air electrode as an electron carrier and catalyst carrier to promote the electrochemical reaction between fuel (such as hydrogen) and oxidant (such as oxygen), and improve the power generation efficiency and performance of fuel cells.
Supercapacitors: Conductive carbon black can improve the conductivity and capacitance of supercapacitor electrodes, enabling them to quickly store and release charges, with higher energy density and power density.
Aerospace and military fields: Used to manufacture antistatic and electromagnetic shielding materials, such as aircraft shell coatings, missile shell materials, etc., to reduce the impact of static electricity on equipment and improve the stealth performance of equipment.
6 crystal forms of calcium carbonate

Calcium carbonate can be divided into cubic, spindle, chain, spherical, flake, needle, etc. according to the crystal form. Different forms of calcium carbonate have different application fields and functions.
Therefore, in order to meet the needs of various industries for different crystalline calcium carbonate products, it is necessary to use crystal form control methods to control the crystallization process of calcium carbonate to produce products with different crystal forms.
1. Cubic calcium carbonate
The so-called cubic refers to calcium carbonate whose crystals are shown as cubes under transmission electron microscope.
Industrial production shows that in the process of producing sodium calcium carbonate using low-temperature technology, without adding any crystal form control agent, controlling the carbonization temperature can obtain a cubic calcium carbonate product. The crystal structure of precipitated calcium carbonate depends largely on the temperature at which it is formed. As long as the temperature at which the crystal nucleus is formed is lower than 30°C, it can be carbonized into cubic calcium carbonate.
2. Rose-shaped and spindle-shaped calcium carbonate
Rose-shaped and spindle-shaped calcium carbonate are generally used in papermaking, rubber, plastic, coating and other industries, especially in high-grade cigarette paper, which can improve the combustion performance and air permeability of cigarette paper.
The main method of producing spindle-shaped calcium carbonate in my country is: at room temperature, the concentration of lime milk is controlled at about 35% (weight ratio), and 30-40% (volume ratio) of CO2 mixed gas is introduced into the reactor for carbonization. The carbonization process is carried out at room temperature. Due to the exothermic reaction, the temperature of the reactor rises from room temperature to about 75°C. The morphology of the product is mainly spindle-shaped, and the particle size is generally a few microns.
3. Chain calcium carbonate
Chain-shaped ultrafine calcium carbonate is composed of several to dozens of fine calcium carbonate grains connected to each other, and has a chain structure. With different synthesis conditions, there will be different particle sizes and aspect ratios.
Chain-shaped ultrafine calcium carbonate has excellent reinforcing effect on natural rubber and synthetic rubber. As reinforcing filler, it can partially replace carbon black or white carbon black, greatly reducing production costs. In addition, chain-like ultrafine calcium carbonate is used as an additive in the coating, papermaking, and plastic industries, showing excellent performance and having broad application prospects.
There are many reports on the synthesis of chain-like calcium carbonate, but the general method is to add a crystal shape controller to control the growth of the crystal nucleus when the Ca (OH) 2 suspension becomes a viscous colloidal emulsion halfway during the carbonization process. The main crystal shape controllers are magnesium salts, potassium salts, sodium polyphosphates, water-soluble metal salts and chelating agents.
4. Spherical calcium carbonate
Due to its good smoothness, fluidity, dispersibility and wear resistance, spherical nano calcium carbonate is widely used in rubber, coating paint, ink, medicine, toothpaste and cosmetics.
Spherical calcium carbonate is usually prepared by low-temperature reaction of calcium salt and carbonate in a concentrated alkaline solution. The main crystal shape controllers are magnesium salts, potassium salts and sodium polyphosphate.
5. Flake calcium carbonate
Flake calcium carbonate is suitable for the papermaking industry and can produce paper with excellent ink absorption, whiteness, printability and smoothness. As a filler and reinforcing agent, flake calcium carbonate has high smoothness, gloss, resistivity and elastic coefficient in the mixture due to its unconventional arrangement.
When flake nano calcium carbonate is used for coated paper pigment, it shows good fluidity and dispersibility, and has better gloss and smoothness than ordinary spindle PCC light calcium carbonate.
6. Needle calcium carbonate
Needle calcium carbonate has a large aspect ratio. It can greatly improve the impact resistance and bending strength of plastics when used as a filler for plastics; the reinforcement effect is more significant when used in rubber.
Application and phase transition of different crystalline alumina

In the fields of mining, ceramics and materials science, aluminum oxide (chemical formula Al2O3), also known as bauxite, is an ionic compound with strong chemical bonds. It has excellent characteristics such as high hardness, high mechanical strength, chemical corrosion resistance, good wear resistance and good thermal conductivity. It is an important chemical raw material in industry.
There are two main ways of arranging the crystal structure of aluminum oxide: one is that the oxygen atoms are arranged in hexagonal stacking, and the other is that the oxygen atoms are arranged in cubic stacking.
(1) Properties and applications of α-Al2O3
α-Al2O3 is commonly known as corundum. α-Al2O3 is a white crystal and is the most common and stable type of alumina crystal. It belongs to the trigonal close-packed structure. In the α-Al2O3 crystal structure, oxygen ions are arranged in a hexagonal close-packed pattern, repeating in two layers of ABABAB... to form several octahedral shapes, while aluminum ions fill in the gaps between each octahedron.
Currently, α-Al2O3 is widely used in abrasive materials, refractory materials, integrated circuit substrates, and structural functional ceramics.
(2) Properties and applications of β-Al2O3
β-Al2O3 is actually an aluminate, which is a composite compound composed of metal oxides and aluminum oxide. Metal ions such as Na+ can diffuse rapidly in this plane layer, so β-Al2O3 crystals can conduct electricity and are an important type of solid electrolyte. Therefore, β-Al2O3 can be used to prepare solid electrolyte diaphragm materials in sodium-sulfur batteries, and can also play an important role in ion conduction and isolating the positive and negative electrodes of the battery.
(3) Properties and applications of γ-Al2O3
γ-Al2O3 is the most commonly used transition state aluminum oxide. It does not exist in nature. In its structure, oxygen ions can be approximated as cubic and closely packed, while aluminum ions are irregularly distributed in the octahedral and tetrahedral voids formed by oxygen ions, belonging to the spinel structure. The preparation process of γ-Al2O3 is relatively simple, and its formation temperature is relatively low, generally in the range of 500~700℃. γ-Al2O3 is insoluble in water but can usually be dissolved in acid or alkali.
Phase transformation of different crystalline alumina
Among different crystalline forms, only α-Al2O3 is a stable crystalline phase, and all other phases are transition phases, which are in a thermodynamically unstable state. As the temperature rises, unstable transitional alumina can be transformed into a stable phase, which is an irreversible transformation of lattice reconstruction.
To obtain stable α-Al2O3, perfect process control is required from the initial ore screening, powder synthesis to sintering. The preparation of high-temperature alumina at home and abroad usually uses industrial aluminum hydroxide or industrial alumina as raw materials, forms a transition phase through dehydration, and then undergoes multiple phase transformations at high temperature, and finally transforms into α-phase Al2O3.
Gibbsite (Al(OH)3) and boehmite (AlOOH) are the most commonly used precursors for the preparation of α-Al2O3. In the initial heat treatment process, aluminum hydroxide transforms into transitional alumina in the form of a metastable structure, and finally ends with the thermodynamically stable phase of α-Al2O3.
In industry, different calcination methods are usually used to transform the metastable phase γ-Al2O3 into the α-stable phase to prepare α-Al2O3 with different morphologies. α-Al2O3 with different morphologies can be produced by controlling different calcination temperatures, adding different types of additives, grinding methods, etc. Usually, α-Al2O3 crystals in various forms such as worm-like, flake-like, columnar, spherical, spherical, and fibrous are included.
With the rapid development of the ceramic industry, pharmaceutical industry, electronic industry and machinery industry, the market demand for alumina still has a lot of room for development, so the research on alumina is of profound significance. Understanding and mastering the crystal structure and properties of alumina is an important prerequisite for the preparation of alumina powder. Different crystalline forms of alumina have different application areas. α-Al2O3 is the most stable of all alumina forms. In production, different types of α-Al2O3 crystals are generally prepared by controlling the growth environment of α-Al2O3 crystals to meet the needs of ceramics, refractory materials and other new functional materials for different crystal microstructures of α-Al2O3.
The main application areas of graphene

(1) Application in the field of electrochemistry
Graphene is a carbon material with a layered grid structure. It has excellent electrical conductivity, chemical stability and thermal stability. It can be used in supercapacitors, lithium-ion batteries, sodium-ion batteries, lead-acid batteries, lithium-sulfur batteries, metal-air batteries, etc.
In the future, how to use cheap raw materials and simple processes to achieve high-quality product production and give full play to the unique structural advantages of graphene for different electrochemical energy storage devices will be a research hotspot.
(2) Application in the field of photocatalytic materials
Graphene has excellent electrical conductivity, electron transport properties, high specific surface area and other properties.
(3) Application in the field of corrosion-resistant coatings
Graphene coatings not only have the cathodic protection of epoxy zinc-rich coatings and the shielding properties of glass flake coatings, but also have excellent adhesion, waterproofness and toughness.
(4) Application in the biomedical field
Because the surface of GO contains a large number of oxygen-containing functional groups (-OH, -COOH, etc.), it can make it have good hydrophilicity, and the two-dimensional GO has good biocompatibility, so it has potential application prospects in biomedical fields such as drug loading and gene delivery.
(5) Application in the field of sensors
Graphene has excellent physical and chemical properties such as high specific surface area, high conductivity and biocompatibility, which is conducive to improving the adsorption capacity of sensitive molecules and increasing the rate of biochemical reactions. These excellent properties make it an ideal candidate material for preparing sensors.
(6) Application in the field of integrated circuits
Graphene has good thermal conductivity and thermal stability, and can be introduced into silicon-based circuits to achieve the purpose of improving rapid heat dissipation.
(7) Application in the field of solar cells
Graphene, as a unique two-dimensional gapless semiconductor, has properties such as high charge carrier mobility and high specific surface area. The prepared film also has high optical transparency, conductivity and flexibility. Therefore, graphene has a wide range of applications in the electron transport layer, hole transport layer, buffer layer, counter electrode, etc. in solar cells.
(8) Application in the field of nanocomposites
Graphene can be combined with other materials of different properties to form composite materials due to its loose porous structure, high conductivity and high material strength. With excellent properties such as high strength, high elastic modulus, high specific surface area and stability, the mechanical properties of materials can be effectively improved or enhanced.
(9) Application in the field of electromagnetic microwave absorption
Graphene not only has a unique physical and chemical structure and excellent mechanical and electromagnetic properties, but also has good microwave absorption properties. In addition, it can be combined with magnetic nanoparticles to prepare a new type of absorbing material. This material has both magnetic loss and electrical loss and has potential application prospects in the fields of electromagnetic shielding and microwave absorption.
(10) Application in other fields
The oxygen-containing functional groups on GO can be adsorbed with the active sites of cotton fabrics. Loading GO on cotton fabrics can effectively improve the antibacterial and UV protection properties of the fabrics.
Graphene is considered to be an ideal hydrogen storage material because of its excellent performance, large specific surface area and ultra-high mechanical strength.
Do you know the 4 degrees of talcum powder?

As a plastic filler, talcum powder can not only save the use of resin, but also significantly improve the physical properties of the product and play a reinforcing role. Talc powder with sufficient fineness can significantly improve the stiffness, impact strength, creep resistance, hardness, surface scratch resistance, heat resistance and heat deformation temperature of the product.
When choosing talc, at least the "four degrees" of talc itself should be considered, namely: purity, whiteness, flakeness and fineness. Generally speaking, in order to evaluate the quality of talc products, at least the above four factors should be considered.
Purity
Purity refers to the talc content of the product. Talc contains impurities in nature and industrial production, and it is impossible for 100% pure talc products to exist in industry. Undoubtedly, the higher the purity of talc powder, the better the reinforcing effect. Some impurities in talc powder not only reduce the purity of talc powder, but also have a significant impact on the performance of the final product.
Whiteness
There are two types of whiteness in the talc industry: narrow whiteness and broad whiteness. Narrow whiteness is a general definition of whiteness, which can be expressed by blue light whiteness R457, Y, L*, Ganz whiteness and Hunter whiteness. Broad whiteness includes dry whiteness, wet whiteness and hue. The so-called wet whiteness is the whiteness measured after adding an appropriate amount of DMP (dimethyl phthalate) to talc powder.
For the same raw materials, the finer the particle size, the higher the whiteness. The higher the moisture content, the lower the whiteness. Although whiteness has no effect on the physical properties of the product, it is very important to maintain the purity of the color for light-colored products.
Flakes
The significant reinforcing effect of talcum powder on plastic products mainly comes from its unique micro-flaky structure. The more complete the flaky structure of talcum powder, the more obvious its reinforcing effect. The two main factors affecting the flake of the product are: the purity of talcum powder and the processing technology of powder.
Impurities in talcum powder do not have a flaky structure. The purer the talcum powder, the fewer impurities and the better the flaky structure. In the process of micronizing the product, the flaky structure of the product is maintained differently when different methods are used. Improper methods and operating conditions may even destroy its flaky structure.
Fineness
Micronization is the development trend of talcum products. The finer the product, the better the reinforcing effect. At the same time, the surface energy of the particles increases, it is easy to agglomerate, difficult to disperse, and expensive. Therefore, we need to choose products with appropriate fineness according to our own technical level and actual needs, not the finer the better.
The evaluation of the particle size of a talc product cannot be based on the average fineness alone. There are at least two indicators to evaluate the quality of a product: D50 and D100 (or D98).
As products become finer and finer, people have higher requirements for the microscopic shape and particle size distribution of fine talc after crushing. The main indicator for evaluating particle size distribution has shifted from D50 to D97, D98 and now D100. At the same time, the reproducibility of particle size distribution is more stringent. When evaluating a product, its average particle size must not only meet the requirements, but more importantly, the particle size distribution should be as narrow as possible, with as few large particles as possible.
The product should strive to achieve the same particle size distribution for each batch, which is very difficult in production practice. In high-end talc products, controlling particle size distribution, especially the number of coarse particles, is a very critical technology, which requires both high-efficiency, high-precision, and reliable grading equipment and rich operating experience and equipment maintenance capabilities. There are only 6-7 companies in China that have mastered relatively mature particle size control technology.
Particle size distribution can be measured by a particle size distribution instrument, including laser method and sedimentation method. However, in production practice, screening method is mostly used to detect the amount of coarse particles.
It is worth noting that talcum powder has a large specific surface area and a small volume density due to its own flaky structure. The volume density of 325 mesh talcum powder is 0.8-0.9g/cm3, while 1250 mesh talcum powder has dropped to 0.25-0.3g/cm3, and 4000 mesh is only about 0.12g/cm3. This causes serious dust pollution during use, difficulty in mixing, increased costs, and reduced yields. In addition, the freight cost of long-distance supply is quite high.