Carbon fiber surface treatment: enhancing composite material performance

Carbon fiber is transformed from organic fiber through a series of heat treatment processes. Its carbon content exceeds 90%. It is an inorganic high-performance fiber and a new material with excellent mechanical properties. Carbon fiber not only inherits the inherent properties of carbon materials, but also combines the flexibility and processability of textile fibers. It is regarded as a new generation of reinforcing fiber and is used in many high-tech fields.
As a reinforcement, although it has a series of excellent performance characteristics, it is also accompanied by some challenges that must be faced. Due to the graphite-like structure, its surface is chemically inert, and it is difficult to infiltrate the resin and react chemically. It is difficult for the surface to combine with the resin, which in turn affects the strength of the composite material. Therefore, it is necessary to treat the surface of the carbon fiber, remove impurities on the surface of the carbon fiber, etch grooves on the surface of the carbon fiber or form micropores to increase the surface area, change the surface properties of the carbon fiber, increase the polar functional groups and surface activation on the surface of the carbon fiber, and then it is easier to infiltrate and react chemically, so that the interface of the composite material is more tightly connected and the strength is increased.
There are many methods for carbon fiber surface treatment, mainly including gas phase oxidation, liquid phase oxidation, electrochemical oxidation, coupling agent coating treatment, plasma treatment, grafting modification technology, etc. Among them, gas phase oxidation is currently the most commonly used method, and electrochemical oxidation is currently the only technology that can be operated online continuously during carbon fiber preparation, and the overall performance of carbon fiber reinforced resin-based composites treated with electrochemical oxidation is improved.
(1) Gas phase oxidation method
Gas phase oxidation methods include air oxidation, ozone oxidation, etc.
Air oxidation method is a method of placing carbon fiber in air with a certain relative humidity for high temperature treatment to oxidize the surface of carbon fiber by high temperature. After oxidation, the non-carbon elements on the surface of carbon fiber increase, which is beneficial to improve the wettability of the fiber and the resin bonding.
(2) Liquid phase oxidation method
Liquid phase oxidation method is to use concentrated nitric acid, concentrated sulfuric acid, hydrogen peroxide and other oxidants to contact carbon fiber for a long time to form carboxyl, hydroxyl and other groups on the fiber surface to enhance the bonding with the resin.
(3) Electrochemical oxidation method
Electrochemical oxidation is a method of treating the surface of carbon fiber by using the conductive properties of carbon fiber as the anode and graphite, copper plate or nickel plate as the cathode under the action of a DC electric field and using different acid, alkali and salt solutions as the electrolyte. The effect of surface electrochemical oxidation treatment is a composite process of layer-by-layer oxidation etching and functional group changes.
(4) Coupling agent coating treatment method
The coupling agent has a double functional group in its chemical structure, which enables it to react chemically with the fiber surface and the resin. Some of the functional groups can form chemical bonds with the fiber surface, while the other functional groups can react chemically with the resin. Through such chemical mediating action, the coupling agent can tightly connect the resin and the fiber surface, thereby enhancing the overall performance of the material. By using a coupling agent, not only can the strength and durability of the material be improved, but also its adhesion and resistance to chemical corrosion can be increased.
(5) Plasma treatment method
Plasma technology mainly uses discharge, high-frequency electromagnetic vibration, shock wave and high-energy radiation to generate plasma under inert gas or oxygen-containing gas conditions to treat the surface of the material.
(6) Grafting modification technology
By grafting the hexagonal nano-pyramids of silicon carbide, the interfacial adhesion between carbon fiber and resin can be significantly enhanced, which not only enhances the mechanical properties of carbon fiber composite materials, but also improves their friction performance. This technology has been applied to the manufacture of brake discs.
By selecting a suitable surface treatment method, the surface properties of carbon fiber can be improved, and its bonding with the matrix material can be enhanced, thereby improving the overall performance of the composite material.
Diamond Micro Powder Development Trend
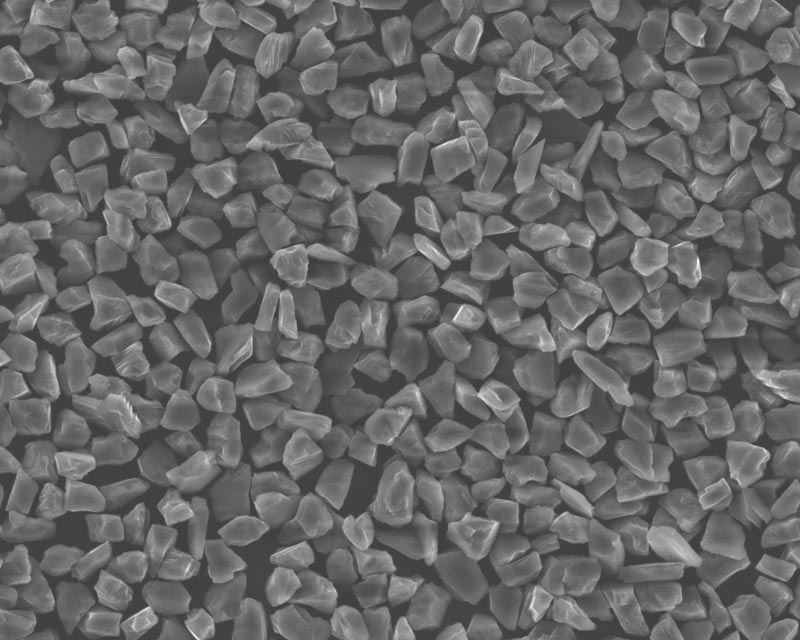
Diamond, commonly known as "diamond drill", is a mineral composed of carbon. It is an allotrope of graphite with a chemical formula of C. It is also the original form of common diamond. Diamond is the hardest substance naturally existing in nature.
Classification of Diamond Micropowder
Diamond micropowder refers to diamond single crystals that are crushed, shaped, purified, and graded to form micron and submicron diamond powder. According to the source of raw materials, it can be divided into natural diamond micropowder and artificial diamond micropowder.
Classification of Diamond Micropowder
Single crystal diamond micropowder is produced by artificial diamond single crystal abrasives, which are crushed and shaped, and produced by special process methods of superhard materials.
The structure of polycrystalline diamond is composed of numerous tiny nano-scale particles bonded by unsaturated bonds, which is very similar to natural black diamond (natural polycrystalline diamond with black or dark gray as the main color).
The role of different types of diamond powder
Traditional diamond powder can be divided into two categories, polycrystalline diamond powder and single crystal diamond powder. With the development of nanotechnology, nano diamond powder has been used and paid more and more attention by people.
Polycrystalline diamond powder
Polycrystalline diamond powder is made from graphite using a unique directional blasting method. The shock wave of the directional blasting of high-explosive explosives accelerates the flying metal flakes and hits the graphite flakes, causing the graphite to be converted into polycrystalline diamond. Polycrystalline diamond powder is characterized by brittleness. Its particle shape is irregular quasi-circular block, and the surface is rough and uneven.
Function: Mainly used in chip optical crystal/ultra-fine processing, large silicon wafer ultra-fine polishing, surface modification and other fields. The spherical polycrystalline diamond powder has a gray-black appearance and a slightly metallic luster.
Single crystal diamond powder
Single crystal diamond powder is produced by static pressure method artificial diamond single crystal abrasive, which is crushed and shaped by special process methods of superhard materials. Its particles retain the single crystal characteristics of single crystal diamond, and its crystal shape is a regular and complete hexahedron, with high strength, toughness and good thermal stability, and strong impact resistance.
Function: Suitable for the manufacture of electroplating products, grinding wheels, grinding wheels, and for polishing, engraving, automotive glass, high-end furniture, ceramics, cemented carbide, magnetic materials, etc. of high-grade stone. It is an ideal raw material for grinding and polishing high-hardness materials such as cemented carbide, ceramics, gemstones, optical glass, etc.
Nanodiamond powder
When the grain size is less than 100nm, it is called nanodiamond. It not only has the excellent properties of diamond, but also has the unique properties of nanomaterials such as small size effect, surface effect, quantum effect, etc. Therefore, it has the dual characteristics of nanomaterials and diamonds and has a wider range of uses.
Function:
(1) Application of fine grinding and polishing. Nanodiamond has the characteristics of both superhard materials and nanomaterials. It can be used in the polishing production of precision parts and for ultra-fine processing of quartz, optical glass, semiconductors, alloys and metal surfaces. The surface roughness value Ra can reach 2-8nm.
(2) Application in the medical field. Nanodiamond can be used as a biological carrier in medical research, and can also be used in wear-resistant coatings on the surfaces of artificial bones and artificial joints to extend the service life of artificial bones and joints.
(3) Application of high thermal conductivity packaging materials. The composite material prepared by adding nanodiamond to a metal high thermal conductivity matrix is expected to become a new type of electronic packaging material with both low thermal expansion coefficient and high thermal conductivity.
Diamond micro powder has a wide range of uses, such as cutting tools, diamond wires, grinding pastes/abrasive fluids, etc. Different application scenarios have different requirements for diamond micro powder, and specialized development is conducive to the development of diamond micro powder. Undoubtedly, diamond micro powder is an indispensable abrasive for the development of products towards high, precise and cutting-edge, and its application prospects are broad and its application fields are also expanding.
In addition to burning cement, what other high-end applications does limestone have?

Limestone is the main raw material for cement production. About 1.4 to 1.5 tons of limestone are consumed to produce 1 ton of cement clinker.
So, in addition to producing cement, what other high-end applications does limestone have?
1. Production of calcium oxide
Calcium oxide is obtained by high-temperature calcination of limestone, commonly known as quicklime, white powder. According to the product appearance, calcium oxide can be divided into block calcium oxide and powdered calcium oxide; according to the different calcium and magnesium content, calcium oxide can be divided into industrial-grade calcium oxide, food-grade calcium oxide, etc. Industrial-grade calcium oxide is divided into four categories: Class I products are for chemical synthesis; Class II products are for calcium carbide; Class III products are for plastics and rubber; Class IV products are for flue gas desulfurization and other uses.
Calcium oxide is an important auxiliary material and basic raw material for steel and plastics. It has huge market prospects in environmental protection fields such as industrial wastewater treatment, garbage incineration, and flue gas desulfurization. As a cost-effective alkaline oxide, calcium oxide is also widely used in highways, high-speed railways, construction, industry (non-ferrous metals, papermaking, sugar making, soda ash, food, medicine, building materials), agriculture and other fields, and is an important basic raw material.
2. Production of calcium hydroxide
Calcium hydroxide is formed by the digestion of calcium oxide and water. Its chemical formula is Ca(OH)2, commonly known as slaked lime and hydrated lime. Its aqueous solution is called clear lime water.
Calcium hydroxide has the general properties of an alkali and is a strong alkali. Since the solubility of calcium hydroxide is much smaller than that of sodium hydroxide and potassium hydroxide, the corrosiveness and alkalinity of its solution are relatively small, so it can be used as an acidity regulator in food to play a role in buffering, neutralization, and solidification. Food-grade calcium hydroxide has a relatively high activity, a relatively loose structure, high purity, good whiteness, low impurity content, and does not contain harmful elements such as Pb and As.
Calcium hydroxide is widely used as a raw material in the calcium preparation production industry, among which calcium gluconate is common. Calcium hydroxide can be used as an acidity regulator in milk powder (including sweetened milk powder) and cream milk powder and its prepared products, and infant formula. Calcium hydroxide can be used as a buffer, neutralizer, and solidifier in beer, cheese, and cocoa products. Due to its pH adjustment and coagulation effects, it can also be used for the synthesis of medicines and food additives, the synthesis of high-tech biomaterials HA, the synthesis of VC phosphates for feed additives, and the synthesis of calcium cyclohexane, calcium lactate, calcium citrate, sugar industry additives and water treatment and other high-end organic chemicals. It is helpful for the preparation of acidity regulators and calcium sources such as edible meat semi-finished products, konjac products, beverage products, and medical enemas.
3. Production of nano calcium carbonate
Nano calcium carbonate refers to functional inorganic fillers with a particle size of 1-100nm, which are widely used in rubber, plastics, papermaking, inks, coatings, sealants and adhesives, medicines, toothpastes, food and other fields.
The industrial production of nano calcium carbonate is mainly based on carbonization. Its raw materials are mainly limestone with a high calcium carbonate content. The powder material products are obtained by calcination, digestion, carbonization, modification, dispersion, and drying.
According to the gradient change of CaO content in limestone, high-quality limestone with a content greater than 54% can be used to produce high-value-added light calcium carbonate and nano calcium carbonate products, which are mainly used in high-end plastics, papermaking, coatings, medicine, electronics, food and other industries; intermediate-quality limestone with a content between 49% and 53% can be used to produce active calcium oxide and calcium hydroxide digested from it, which are mainly used in metallurgical solvents, chemicals and food deep processing industries; low-quality limestone with a content less than 48% can be used in the cement industry and the construction industry.
According to the different calcium oxide content of limestone resources, the limestone raw materials are distributed to various related industries in a tiered manner, so as to achieve a fully closed industrial chain with high-quality resources, full utilization, and maximum value and environmental effects.
Development of graphene-modified thermosetting resins

Graphene is a honeycomb two-dimensional planar material composed of a single layer of carbon atoms connected in an sp2 hybrid manner. It has many excellent properties, such as high carrier mobility, high light transmittance, high specific surface area, high Young's modulus, high fracture strength, etc. These properties make graphene an ideal filler for improving the performance of thermosetting resins. Thermosetting resin materials have attracted widespread attention from industry and academia due to their advantages such as high specific strength, large specific modulus, good thermal stability and corrosion resistance.
There are two main ways to modify the surface of graphene powder: covalent bond modification and non-covalent bond modification.
Covalent bond modification is a method that uses chemical reactions to achieve covalent bonding of modifiers on the graphene surface, or special treatment of graphene to form new functional groups or chemical bonds, thereby improving the compatibility and dispersibility of graphene powder in the resin matrix.
Non-covalent bond modification mainly combines the modified group with graphene through π-π bond stacking to achieve effective modification of graphene. The advantage of this method is that it improves the dispersibility of graphene without changing the chemical structure of graphene or introducing new covalent bonds.
For different types of thermosetting resin matrices, it is necessary to select a suitable modification method so that the graphene powder can be evenly dispersed in the resin without affecting the performance of the resin matrix.
As a new type of reinforcing filler, graphene can be evenly dispersed in the thermosetting resin matrix to significantly improve the mechanical properties, ablation resistance, electrical properties, corrosion resistance and wear resistance of the composite material, thereby expanding the application range of thermosetting resin-based composite materials.
Mechanical properties
Graphene can significantly improve the mechanical properties of thermosetting resin materials, making composite materials have important application value in the fields of machinery and automotive structural parts.
Anti-ablation performance
The addition of graphene oxide will improve the thermal conductivity of the composite material and accelerate the heat extraction, reducing the linear ablation rate of the composite material by 62.08%. The addition of graphene oxide is conducive to inducing the formation of a carbon layer in the matrix during the ablation process, enhancing the degree of graphitization of the matrix, and forming a heat insulation layer to prevent heat from expanding into the material, thereby reducing the linear ablation rate of the composite material and improving the ablation resistance of the resin composite material.
Electrical properties
Graphene is a carbon material with a two-dimensional honeycomb lattice structure composed of sp2 hybridized carbon atoms. The excellent structural π electrons provide a conjugated effect, which greatly improves the mobility of electrons. At the same time, under ideal conditions, the conduction band and valence band of graphene are in contact at the Dirac point, so that electrons can move between the valence band and the conduction band without energy hindrance, thereby promoting graphene to have excellent electrical properties.
Corrosion resistance
Thermosetting resin is a common matrix material in coating materials and has excellent corrosion resistance, but the cured resin material will produce micropores or microgaps, which weakens the protection ability of the substrate. The chemical stability and barrier properties of graphene itself can effectively prevent the penetration of corrosive agents and prevent further diffusion of corrosive agents in the surface when they reach the metal surface, minimizing the degree of corrosion damage to the protective substrate, making it the preferred filler for metal substrate coatings.
Application of graphene-modified thermosetting resin
At present, graphene-modified thermosetting resin is mainly used in heavy-duty anti-corrosion coatings, sprayed on large equipment (such as large ships, surface platforms, wind turbines, etc.) to prevent corrosion and extend service life; in the future, graphene-modified thermosetting resin will also be more widely used in aerospace, electronic components and other fields.
Application of modified silica powder

Silica powder is a very important inorganic non-metallic functional filler that can be compounded with organic polymers and improve the overall performance of composite materials. It is widely used in electrical and electronic, silicone rubber, coatings, adhesives, potting materials and other fields.
Silica powder itself is a polar, hydrophilic substance. It has different interface properties from the polymer matrix, poor compatibility, and is often difficult to disperse in the base material. Therefore, in order to make the composite material more excellent, it is usually necessary to modify the surface of silica powder and purposefully change the physical and chemical properties of the surface of silica powder according to the needs of the application, so as to improve its compatibility with organic polymer materials and meet its dispersion and fluidity requirements in polymer materials.
Copper clad laminate
Copper clad laminate is an electronic basic material made by impregnating glass fiber or other reinforcing materials with a resin matrix, adding different fillers, and covering one or both sides with copper foil through processes such as glue adjustment and impregnation, and then hot pressing. The addition of modified silica powder can reduce the production cost of copper clad laminates and improve their heat resistance, conductivity and mechanical properties.
Rubber
Rubber is a highly elastic polymer material with reversible deformation. It can be widely used in electronics, automobiles, civil engineering, national defense, medical and health, and daily necessities. In the process of rubber preparation, adding a certain amount of inorganic filler can not only reduce the production cost of rubber, but also significantly improve the comprehensive physical properties and dynamic mechanical properties of rubber composite materials.
Plastic
Silicon powder can be used as a filler in materials such as polyethylene (PE), polyvinyl chloride (PVC), polypropylene (PP), polyphenylene ether (PPO) in the process of making plastics. It is widely used in many fields such as construction, automobiles, electronic communication insulation materials, agriculture, daily necessities, national defense and military.
Epoxy molding compound
Epoxy molding compound is a molding compound made of a variety of additives. It is a key material for electronic packaging and accounts for more than 97% of the market for microelectronic packaging. It can be widely used in semiconductors, consumer electronics, integrated circuits, aviation, military and other packaging fields.
Epoxy casting
Epoxy insulation casting material is a liquid or viscous polymerizable resin mixture made of resin, curing agent, filler, etc. At the pouring temperature, the castable has good fluidity and less volatiles, fast curing, and small shrinkage after curing. The epoxy resin formed after the castable is an insulating product that integrates multiple functions such as insulation, moisture-proof, mildew-proof, anti-corrosion, fixation and isolation.
Electronic potting glue
Potting glue is often used in electronic components, mainly for bonding, sealing, barrier and protection. It is liquid before curing and has a certain fluidity. The viscosity of the glue varies according to the material, performance and production process of the product, and its use value can only be realized after the glue is completely cured.
Artificial quartz stone
Silicon powder is used as a filler in artificial quartz stone, which can not only reduce the consumption of unsaturated resin, but also improve the wear resistance, acid and alkali resistance, mechanical strength and other properties of artificial quartz plate.
Different application fields of silicon micropowder have different quality requirements. Therefore, when choosing the application of silicon micropowder, it should be combined with the needs of downstream industries, and comprehensive cost, efficiency, performance and other factors should be considered to select the appropriate silicon micropowder type and modifier and formula. With the continuous improvement of my country's economy and society, at present, the application research of modified silicon micropowder will mainly focus on high-end copper clad laminates, high-performance adhesives, insulation materials and other high-tech fields produced with spherical silicon micropowder as raw materials. Refinement and functional specialization will be the mainstream direction of modified silicon micropowder application in the future.
Common powder surface modification equipment

Factors that affect the powder modification effect include the properties of the powder raw materials, modification methods, modification processes, modifiers and their formulas, and modification equipment. When the powder modification process and modifier or formula are determined, the modification equipment becomes the key factor affecting the powder modification effect.
Powder modification equipment mainly undertakes three responsibilities: one is mixing, the second is dispersion, and the third is that the modifier melts in the equipment and combines well with the powder. In addition, the powder modification equipment is also required to have less energy consumption and wear, no dust pollution, simple equipment operation, and stable operation.
1. HEM high-efficiency hybrid modifier
The HEM high-efficiency hybrid modifier has six groups of stirring paddles, 24 moving knives and guide plates. The materials are fully mixed repeatedly in the bin and repeatedly act with the additives, so that the materials absorb the additives, so that the additives are evenly coated on the surface of the powder.
2. High-speed heating mixer
The high-speed heating mixer is one of the commonly used equipment for chemical coating and modification of inorganic powders, such as inorganic fillers or pigments. It is a mixing equipment widely used in the plastic products processing industry.
3. SLG continuous powder surface modifier
The SLG continuous powder surface modifier is mainly composed of a thermometer, a discharge port, an air inlet, an air duct, a main machine, a feed port, a metering pump and a feeder.
4. High-speed airflow impact surface modifier
The main structure is mainly composed of high-speed rotating rotor, stator, circulation loop, wing, jacket, feeding and discharging device. The whole system consists of mixer, metering feeding device, high-speed airflow impact surface modifier, product collection device, control device, etc.
5. Horizontal paddle mixer
The horizontal paddle mixer is an intermittent powder surface modifier with horizontal cylinder and single-axis multi-paddle as structural characteristics. It is mainly composed of transmission mechanism, main shaft, cylinder, end cover, etc.
6. Turbine (rotary) mill
It is mainly composed of machine base, drive part, crushing chamber, gap adjustment and inlet and outlet. The characteristic is that the heat generated by the ultrafine grinding process (50℃~60℃) is used to introduce the crushed ultrafine powder into the vortex mill, and the pre-heated and melted stearic acid modifier is metered to carry out continuous surface modification.
7. Turbo mill
The Turbo mill is mainly composed of a depolymerization wheel, a discharge door, an air inlet, a classifier, a feed port, a multi-channel surface dispersant inlet and a feeder.
Finally, the selection principles of surface modification equipment are summarized as follows:
(1) Good dispersibility of powder and surface modifier. Only with good dispersibility can the powder and surface modifier have a relatively equal opportunity and effect, and the amount of surface modifier can be reduced.
(2) The modification temperature and residence time are adjustable within a certain range.
(3) Low energy consumption per unit product and low wear. In addition to the modifier, the main cost of surface modification is energy consumption. Low-energy modification equipment can reduce production costs and improve product competitiveness; low wear can not only avoid the contamination of modified materials, but also improve the operation efficiency of the equipment and reduce operating costs.
(4) Less dust pollution. The escape of dust during the modification process not only pollutes the production environment, but also causes material loss, resulting in increased product production costs. Therefore, the dust pollution of the equipment must be investigated.
(5) Continuous production, simple operation, and low labor intensity.
(6) Smooth and reliable operation.
(7) High level of automatic control, which can automatically adjust the processing volume, modifier addition amount, modification temperature, residence time and other factors according to the properties of the material and the properties of the surface modifier.
(8) The production capacity of the equipment should be consistent with the designed production scale. When the designed production scale is increased, large-scale equipment should be selected as much as possible to reduce the number of equipment to reduce the floor space, production costs and facilitate management.
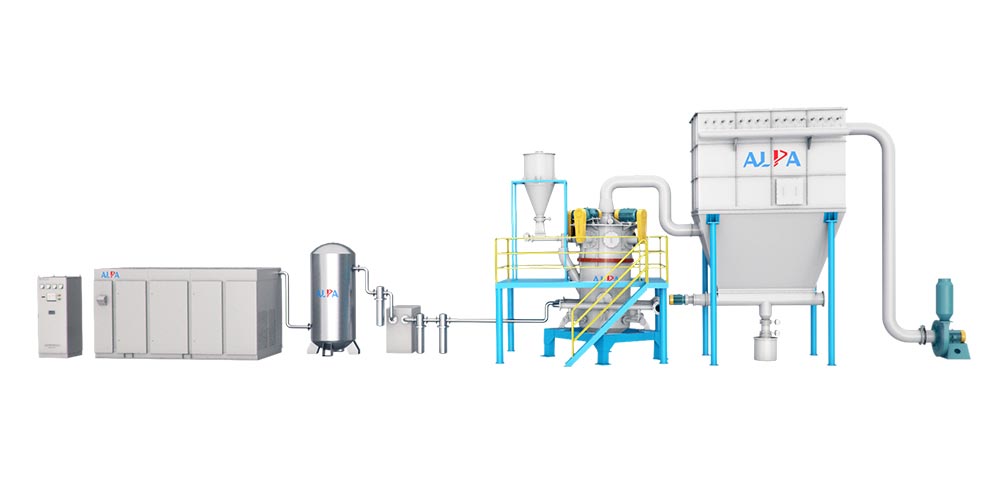
Learn about general powder processing equipment production line
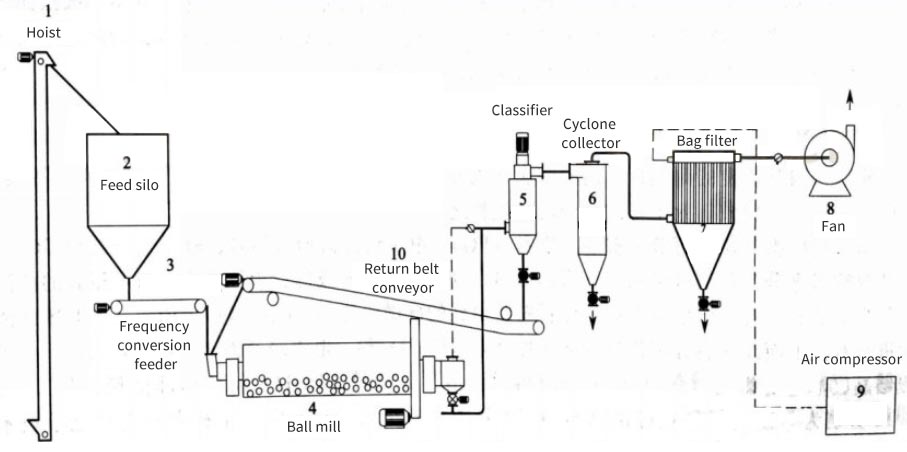
Powder processing equipment is an indispensable core component in modern industrial production. They run through multiple key process flows such as powder raw material transportation, grinding, classification, surface treatment, solid-solid separation, liquid-solid separation, gas-solid separation, drying, mixing, granulation, molding, roasting/calcining, cooling, packaging, and warehousing.
Feeding/Feeding: Vibrating feeder, Electromagnetic vibrating feeder, Screw feeder, Disc feeder, Rotary feeder
Conveying: Belt conveyor, Chain conveyor, Bucket elevator, Pneumatic conveyor, Hydraulic conveyor, Screw conveyor
Commonly used industrial powder and particle conveying equipment
1 Screw conveyor
2 Pipe chain conveyor
3 Positive pressure pneumatic conveying equipment
Grinding Mill
Jaw crusher: uses the movable jaw to periodically approach and leave the fixed jaw to crush materials.
Cone crusher: uses the swinging movable cone to periodically approach and leave the fixed cone to crush materials.
Hammer crusher: uses the impact generated by the rotation of the hammer head hinged on the rotor to crush materials.
Impact crusher: uses the impact of the plate hammer rigidly fixed on the rotor and the impact plate to crush materials.
Shear crusher: uses the relatively fast movement between the moving and static sharp blades to crush materials.
Roller mill: uses synchronously rotating extrusion rollers to crush materials.
Impact mill: uses horizontal high-speed rotating impellers to make materials move centrifugally at high speed, and collide and crush each other in the vortex chamber.
Ball mill/tube mill: uses the impact, grinding, and shearing of the grinding media in the rotating cylinder to crush materials. The grinding media are spherical, short columnar, rod-shaped, etc.
Screening mill: Use a mill with a screening mechanism to crush and classify the crushed materials.
Vibration mill: Use the impact, grinding and shearing of the grinding media in the vibrating cylinder to crush the material.
Tower mill/vertical stirred mill: Use the impact, grinding and shearing of the grinding media driven by the vertical stirring mechanism to crush the material.
Horizontal stirred mill: Use the impact, grinding and shearing of the grinding media driven by the horizontal stirring mechanism to crush the material.
Vertical mill/wheel mill: Use the relative rotation of the grinding disc and the grinding roller to grind and crush the material, and classify the ground material, such as Raymond mill, Loesche mill, etc.
Ring roller mill: Use the revolution and rotation of the grinding ring (roller) to crush the material between the grinding ring and the grinding circle by impact, collision, shearing.
Horizontal roller mill: The rotating cylinder forces the material to be clamped between the cylinder wall and the high-pressure roller, and is repeatedly squeezed, ground, sheared and crushed.
Planetary mill: Use the impact and grinding of the grinding media driven by the revolution and rotation of the grinding cylinder to crush the material.
Colloid mill: The material is sheared and ground between the high-speed rotating teeth and fixed teeth and is effectively emulsified and dispersed.
Airflow pulverizer: The material is crushed by strong collision, impact and friction between the materials or between the materials and the wall of the device using high-speed airflow.
Heavy-duty grinder: The disc-shaped roller runs along the bottom track, repeatedly applying rolling and shearing to crush the material.
Sidewall grinder: The cylindrical roller is driven by the rotating shaft to rotate and the side wall produces an extrusion effect to crush the material.
Classifying
Screening machine: Classification is performed using screens, including horizontal screens, vibrating screens, resonance screens, drum screens, etc.
Fixed screen: Classification is performed using an inclined screen plate composed of parallel grid bars.
Gravity sedimentation classifier: Classification is performed using the difference in the final settling speed of particles in the fluid.
Cyclone: Under the action of centrifugal force, larger particles are thrown to the wall of the device and rotate downward to be discharged, and smaller particles rotate upward to be discharged to achieve classification.
Centrifugal powder classifier: uses the different movement trajectories of particles in the centrifugal field to achieve gas-solid separation or powder classification.
Cyclone powder classifier: uses a turntable to drive the blades to rotate for powder classification.
Rotor classifier: When the gas-solid two-phase flow passes through the gap between the blades of the high-speed rotor, large particles are thrown out in the direction of centrifugal force, thereby classifying.
Dispersion classifier: The material is dispersed and scattered in the dispersion area and then enters the classification area.
Surface modification (activation) of talc and its application in plastics and coatings

Talc is a hydrated silicate with a chemical formula of 3MgO·4SiO2·H2O. Its crystal shape can be flake, leaf, needle and block.
The structure of pure talc consists of a layer of brucite (magnesium hydroxide, MgO·H2O) sandwiched between two layers of silica, with the layers stacked on top of each other and adjacent talc layers bonded by weak van der Waals forces. When shearing is applied to it, the layers can easily slide against each other.
Talc is inert to most chemical reagents, does not decompose when in contact with acid, is a poor conductor of electricity, has low thermal conductivity and high thermal shock resistance, and does not decompose when heated to 900°C.
These excellent properties of talc make it a good filler and are widely used in the fields of plastics and coatings, but the hydrophilic surface of talc limits its application in some hydrophobic fields. In order to further improve its performance and expand its application areas, surface modification is necessary.
1. Surface modification methods and commonly used modifiers for talc
(1) Commonly used surface modifiers for talc
In order to make talc better bonded with polymers, there are two main types of modifiers currently used for modification:
Coupling agents: mainly titanates, aluminates, silanes and stearic acids. Titanates are more commonly used. Their molecular structure is R´-O-Ti-(O-X-R-Y)n, where R´O- can react with the chemical structure of the filler surface, R is a long-chain entangled group with a fat or aromatic structure, which can improve the compatibility between the polymer and the filler, and Y is an active reactive group that can crosslink or bond in the polymer filling system.
Surfactants: mainly sodium dodecylbenzene sulfonate, sodium dodecyl sulfonate, dodecyltrimethylammonium bromide, dodecyltrimethylammonium chloride, sodium olefin sulfonate, etc., which have the same effect as coupling agents in improving the compatibility between polymers and fillers, but their mechanism of binding to the filler surface is different from that of coupling agents.
(2) Surface modification methods of talcum powder
Surface coating modification: Covering the surface of the particles with surfactants to give the particles new properties is a common method nowadays.
Mechanochemical method: A modification method that uses crushing, friction and other methods to enhance surface activity. This method is to crush and rub relatively large particles to make them smaller.
External film modification: A method of uniformly coating a layer of polymer on the surface of the particles to change the surface properties of the particles. For talcum powder, it can be first crushed and activated, then adsorbed with surfactants under certain conditions, and then adsorbed with monomers through surfactants, and finally the monomers undergo polymerization to achieve the effect of surface coating.
Local active modification: Use chemical reactions to form different functional groups on the surface of particles to achieve the purpose of surface modification.
High-energy surface modification: Use high-energy discharge, ultraviolet rays, plasma rays, etc. to modify the surface of particles. This method uses the huge energy generated by high-energy discharge, ultraviolet rays, plasma rays, etc. to modify the surface of particles, making their surfaces active. Improve the compatibility of particles and polymers.
Precipitation reaction modification: modification using precipitation reaction. This method uses the precipitation effect to coat the surface of particles to achieve the modification effect.
2. Application of talcum powder in the field of plastics
Talc powder fills plastics to improve the rigidity, dimensional stability, and lubricity of products, prevent high-temperature creep, reduce wear on molding machinery, and make the polymer improve hardness and creep resistance through filling while the impact strength remains basically unchanged. If handled properly, it can improve the heat shock resistance of polymers, improve the molding shrinkage of plastics, the bending elastic modulus and tensile yield strength of products.
Application in PP materials: This application is the most widely studied and most widely used. It is now widely used in automotive parts, such as automotive bumpers, engine peripheral parts, air conditioning parts, dashboards, headlights, chassis, pedals and other parts.
Application in automobiles: PP materials have a wide range of sources, low density, and can be modified to improve their physical and chemical properties. It can reduce costs, reduce weight, and reduce fuel consumption without reducing mechanical properties. For example, the automotive cooling fan injected with PP materials filled with talcum powder is not only light in weight and low in noise, but also improves cooling efficiency.
23 Application Fields of Kaolin

(1) Ceramic industry
The ceramic industry is the earliest industry to use kaolin and the industry with the largest amount of kaolin. The general amount is 20% to 30% of the formula. The role of kaolin in ceramics is to introduce Al2O3, which can improve its chemical stability and sintering strength.
(2) Rubber
Filling kaolin into the colloidal mixture of rubber can enhance the chemical stability, wear resistance and mechanical strength of the rubber, prolong the hardening time, and improve the rheological properties, mixing properties and vulcanization properties of the rubber, increase the viscosity of the unvulcanized product, and prevent it from sinking, collapsing, sagging, deformation, flat tubes, etc.
(3) Paint pigments
Kaolin has been used as a filler for paints and varnishes for a long time because of its white color, low price, good fluidity, stable chemical properties and large surface cation exchange capacity.
(4) Refractory materials
Kaolin has good refractory properties and is often used to produce refractory products.
(5) Catalysts
Kaolin can be used directly or after acid or alkali modification as a catalyst matrix, or it can be synthesized into molecular sieves or catalysts containing Y-type molecular sieves through in-situ crystallization technology.
(6) Cable materials
The production of high-insulation cables requires the addition of excessive amounts of electrical performance improvers.
(7) Lubrication field
Kaolin has a layered structure and small particle size, which makes it have good lubricity.
(8) Heavy metal wastewater treatment
Kaolin has abundant reserves, wide sources and low prices. Its natural two-dimensional layered structure gives it a large specific surface area and good adsorption performance.
(9) Secondary resource utilization
Modified kaolin is also used in the field of secondary resource utilization to recover metal ions.
(10) Treatment of degraded oil products
Currently, the most commonly used method for treating degraded oil products is adsorption regeneration, which is mainly made of silica-alumina adsorbents made from processed bentonite, kaolin, etc.
(11) Building phase change thermal storage materials
Using dimethyl sulfoxide (DMSO) as an intercalation agent, the coal-based kaolin was intercalated and modified by the melt intercalation method, and the intercalated kaolin was used as the matrix.
(12) Solar energy storage materials
Using kaolin and sodium stearate as raw materials, a new type of kaolin/sodium stearate phase change heat storage material is prepared.
(13) Molecular sieves
Kaolin is abundant in reserves, cheap in price, and has a high aluminum-silicon content, making it a good raw material for preparing molecular sieves.
(14) Kaolinite organic intercalation materials
The intercalation method generally involves inserting organic molecules or layered polymers into layered inorganic materials to prepare intercalation composite materials.
(15) Nanomaterials
Due to their special size, nanomaterials have many unique properties, such as shielding ultraviolet rays and electromagnetic waves, and are used in military, communication, computer and other industries; adding nanoclay in the production process of water dispensers and refrigerators has antibacterial and disinfecting effects; adding nanoclay in ceramic production can increase the strength of ceramics by 50 times, and can be used to manufacture engine parts.
(16) Preparation of glass fiber
Kaolin is an important raw material for the preparation of glass fiber, providing Al2O3 and SiO2 for glass fiber.
(17) Mesoporous silica materials
Mesoporous materials are materials with pore sizes of 2 to 50 nm. They have large porosity, adsorption capacity and specific surface area.
(18) Hemostatic materials
Uncontrolled bleeding after trauma is the main cause of high mortality. Based on the natural hemostatic agent daizheshi's ability to control bleeding, a new type of iron oxide/kaolin nanoclay composite material was successfully synthesized.
(19) Drug carrier
Kaolin is a 1:1 layered crystal with a tight and uniform arrangement and a large specific surface area. It is often used as a sustained-release material.
(20) Antibacterial material
(21) Tissue engineering
Using kaolin as a binder, a three-dimensional MBG scaffold with excellent mechanical strength, mineralization ability and good cell response was successfully prepared using a modified polyurethane foam (PU) template method.
(22) Cosmetics
Kaolin can be used as an additive in cosmetics to enhance oil and water absorption, enhance the affinity of cosmetics to the skin, and improve the moisturizing function.
(23) Application of kaolin in the papermaking industry
In the papermaking industry, the international market for kaolin is relatively prosperous, and its sales volume exceeds that of ceramics, rubber, paint, plastics, refractory materials and other industries.
Surface modification of graphite anode materials

Graphite is the first negative electrode material for lithium-ion batteries to be commercially applied. After three decades of development, graphite is still the most reliable and widely used negative electrode material.
Graphite has a good layered structure, with carbon atoms arranged in a hexagonal shape and extending in a two-dimensional direction. As a negative electrode material for lithium-ion batteries, graphite has high selectivity for electrolytes, poor high current charge and discharge performance, and during the first charge and discharge process, solvated lithium ions will be inserted into the graphite interlayers, reduced and decomposed to produce new substances, causing volume expansion, which can directly lead to the collapse of the graphite layer and deteriorate the cycle performance of the electrode. Therefore, it is necessary to modify graphite to improve its reversible specific capacity, improve the quality of the SEI film, increase the compatibility of graphite with the electrolyte, and improve its cycle performance. At present, the surface modification of graphite negative electrodes is mainly divided into mechanical ball milling, surface oxidation and halogenation treatment, surface coating, element doping and other means.
Mechanical ball milling method
Mechanical ball milling method is to change the structure and morphology of the graphite negative electrode surface by physical means to increase the surface area and contact area, thereby improving the storage and release efficiency of lithium ions.
1. Reduce particle size: Mechanical ball milling can significantly reduce the particle size of graphite particles, so that the graphite negative electrode material has a larger specific surface area. Smaller particle size is conducive to the rapid diffusion of lithium ions and improves the rate performance of the battery.
2. Introduce new phases: During the ball milling process, graphite particles may undergo phase changes due to mechanical forces, such as the introduction of new phases such as rhombohedral phases.
3. Increase porosity: Ball milling will also produce a large number of micropores and defects on the surface of graphite particles. These pore structures can serve as fast channels for lithium ions, improving the diffusion rate of lithium ions and the charge and discharge efficiency of the battery.
4. Improve conductivity: Although mechanical ball milling itself does not directly change the conductivity of graphite, by reducing the particle size and introducing a pore structure, the contact between the graphite negative electrode and the electrolyte can be more sufficient, thereby improving the conductivity and electrochemical performance of the battery.
Surface oxidation and halogenation treatment
Oxidation and halogenation treatment can improve the interfacial chemical properties of graphite negative electrode materials.
1. Surface oxidation
Surface oxidation usually includes gas phase oxidation and liquid phase oxidation.
2. Surface halogenation
Through halogenation treatment, a C-F structure is formed on the surface of natural graphite, which can enhance the structural stability of graphite and prevent the graphite flakes from falling off during the cycle.
Surface coating
The surface coating modification of graphite negative electrode materials mainly includes carbon material coating, metal or non-metal and its oxide coating, and polymer coating. The purpose of improving the reversible specific capacity, first coulomb efficiency, cycle performance and high current charge and discharge performance of the electrode is achieved through surface coating.
1. Carbon material coating
A layer of amorphous carbon is coated on the outer layer of graphite to make a C/C composite material with a "core-shell" structure, so that the amorphous carbon contacts the solvent, avoids direct contact between the solvent and the graphite, and prevents the graphite layer exfoliation caused by the co-embedding of the solvent molecules.
2. Metal or non-metal and their oxide coating
Metal and its oxide coating is mainly achieved by depositing a layer of metal or metal oxide on the surface of graphite. Coating metal can increase the diffusion coefficient of lithium ions in the material and improve the rate performance of the electrode.
Non-metal oxide coating such as Al2O3, amorphous Al2O3 coating the graphite surface can improve the wettability of the electrolyte, reduce the diffusion resistance of lithium ions, and effectively inhibit the growth of lithium dendrites, thereby improving the electrochemical properties of graphite materials.
3. Polymer coating
Inorganic oxides or metal coatings are brittle, difficult to coat evenly, and easily damaged. Studies have shown that graphite coated with organic acid salts containing carbon-carbon double bonds is more effective in improving electrochemical performance.